Table of Contents
Table of Content ............................................................................................................................ 1
Preface ............................................................................................................................................ 4
Acknowledgements ....................................................................................................................... 4
Contacts and Comments ........................................................................................................................... 4
Principal Authors (USDA/FSIS/OPHS/Science Staff) ............................................................................. 4
Executive Summary ...................................................................................................................... 5
Acronyms ....................................................................................................................................... 7
Introduction ................................................................................................................................... 8
Overview of the Sampling Plans ................................................................................................ 11
Domestic Sampling Plan ......................................................................................................................... 11
Import Reinspection Sampling Plan ....................................................................................................... 13
Policy and procedures for holding or controlling product under NRP ................................................... 14
Domestic Scheduled Sampling Program ................................................................................... 15
Summary of Domestic Residue Sampling Program ................................................................ 16
Table 1. FY 2016 Tier I and II List of Animal Class by Method/Chemical Class (Analyses Performed)
................................................................................................................................................................ 16
Table 2. FY 2016 Number of Scheduled Residue Samples Tested, by Animal Class ............................ 17
Table 3. FY 2016 NRP Domestic Scheduled Samples Analyzed by Animal Class – and Summary
Results ..................................................................................................................................................... 18
Table 4. FY2016 NRP Residue Scheduled Samples -Number of Residue Samples Tested Per Chemical
Method by Animal Class ........................................................................................................................ 19
Table 5. FY 2016 NRP Residue Scheduled Samples - Number of Chemical Analytes Tested Per
Chemical Method by Animal Class ........................................................................................................ 20
Table 6. FY 2016 Domestic Scheduled Sampling Plan Violations ........................................................ 21
Table 6. FY 2016 Domestic Scheduled Sampling Plan Violations – Federal Plants .............................. 22
Summary of Domestic Inspector -Generated Sampling Program ......................................... 23
Table 7. FY 2016 Tier II Inspector Generated Sampling (KIS
TM
) Test ................................................. 24
Table 8. FY 2016 Tier II Inspector-Generated Sampling (COLLGEN/ STATE/ SHOW) Projects ....... 25
2
Table 9. FY 2016 Number of Residue Violations results in Inspector Generated Sampling by Chemical
Residue and Animal Class ( include KIS ™ test, COLLGEN/ STATE/ SHOW project codes) ............ 26
Table 9. FY 2016 Number of Residue Violations results in Inspector Generated Sampling by Chemical
Residue and Animal Class ( include KIS ™ test, COLLGEN/ STATE/ SHOW project codes) (cont.)
................................................................................................................................................................ 27
Table 10. FY 2016 Number of Non-Violative results in Inspector Generated Sampling by Chemical
Residue and Animal Class ( include KIS ™ test, COLLGEN/ STATE/ SHOW project codes) ........... 28
Table 10. FY 2016 Number of Non-Violative results in Inspector Generated Sampling by Chemical
Residue and Animal Class ( include KIS ™ test, COLLGEN/ STATE/ SHOW project codes) (cont.) 29
Import Residue Reinspection Sampling Program ................................................................... 30
Table 11. FY 2016 NRP Import Residue Samples - Number of Residue Samples Tested Per Chemical
Method by Production Class and Product Type ...................................................................................... 31
Table 12. FY 2016 Number of Import Residue Samples by Inspection Level, per Exporting Country
and Production Type ............................................................................................................................... 32
Table 13. FY 2016 Number of Import Residue Samples Analyzed, by Exporting Country and
Production Type ...................................................................................................................................... 33
Table 13. FY 2016 Number of Import Residue Samples Analyzed, by Exporting Country and
Production Type (Cont.) ......................................................................................................................... 34
Table 14. FY 2016 Number of Chemical Analyates Tested Per Exporting Country and Production Type
................................................................................................................................................................ 35
Table 14. FY 2016 Number of Chemical Analyates Tested Per Exporting Countries and Production
Type (Cont.) ............................................................................................................................................ 36
Table 15. FY 2016 Number of Samples and Chemical Residues under the Import Residue Sample
Program, by Exporting Country .............................................................................................................. 37
Table 16. FY 2016 Import Residue Sample Program (Non-Violative and Violative) Results, by
Exporting Countries, Chemical Residues and Production Class ............................................................ 38
Appendix I ................................................................................................................................... 39
NRP Non-Violative Positive and Violative Residue Samples Results ................................................... 39
Appendix II .................................................................................................................................. 39
Statistical Table ....................................................................................................................................... 39
Appendix III ................................................................................................................................ 41
List of Chemical Residues by Class/Method .......................................................................................... 41
Appendix IV ................................................................................................................................ 46
U.S. NRP – Domestic Scheduled Sampling Program ............................................................................. 46
3
Appendix V .................................................................................................................................. 46
U.S. NRP – Import Re-inspection Sampling Program ............................................................................ 46
Appendix VI ................................................................................................................................ 47
NRP – Domestic Inspector Generated Sampling Program (include KIS™ test) & lab confirmed residue
results ...................................................................................................................................................... 47
Appendix VII ............................................................................................................................... 48
2016 FSIS Residue Sampling for Siluriformes ....................................................................................... 48
Table 17. FY2016 NRP Residue Scheduled Samples -Number of Residue Samples Tested Per
Chemical Method by Sampling Plan ...................................................................................................... 48
Table 18. FY 2016 NRP Residue Scheduled Samples - Number of Chemical Analytes Tested Per
Chemical Method by Sampling Plan ...................................................................................................... 49
Table 19. FY 2016 NRP Siluriformes Residue Inspection Program Violations ..................................... 49

4
Preface
The “2016 Food Safety and Inspection Service (FSIS) National Residue Program Data” publication (the
‘Red Book’) explains FSIS’ chemical residue sampling plans and presents National Residue Program
(NRP) testing results by fiscal year. [For those reading this electronically, this document has been
commonly known as the “Red Book” because the covers of the printed versions are red.] In addition, the
following appendices are included for the convenience of the reader: Appendix I, NRP Positive Non-
Violative and Positive Violative Residue Samples Results; Appendix II, Statistical Table; Appendix III,
FY2016 List of Chemical Residues by Class/Method ;Appendix IV, Summary of Scheduled Sampling
Data from 2013 to 2016, Appendix V, Summary of Import Re-inspection Sampling Data from 2013 to
2016 and Appendix VI, Inspector Generated Sampling Data from 2013 to 2016 (includes KIS™ test)
Acknowledgements
We would like to extend our gratitude to the thousands of FSIS field inspection personnel who collected
and submitted the residue samples and to all the laboratory staff who prepared the residue samples for
analysis, analyzed the residue samples and documented the results from the analysis of the residue
samples. We would like to acknowledge the Office of Data Intergration and Food Protection (ODIFP)
members for providing the data.
Contacts and Comments
Personnel from the Science Staff (SciS), within the Office of Public Health Science (OPHS) at the United
States Department of Agriculture’s (USDA) Food Safety and Inspection Service (FSIS) coordinated this
effort and are responsible for the publication of this material. Questions about the U.S. NRP should be
directed to:
USDA/FSIS/OPHS
1400 Independence Avenue, SW
355 E Street - Patriot Plaza III
Washington, D.C. 20250-3700
Questions can be sent to askFSIS:
http://askfsis.custhelp.com/app/utils/login_form/redirect/ask
Principal Authors (USDA/FSIS/OPHS/Science Staff)
Naser Abdelmajid
Randolph Duverna

5
Executive Summary
The United States National Residue Program (NRP) is comprised of the following programs:
• Domestic Sampling Plan
o Scheduled
o Inspector-Generated
• Import Reinspection Sampling Plan
During FY 2016, (October 2015 to September 2016), FSIS reported 922 residue violations 29 stemmed
from the Domestic Scheduled Sampling Program and 893 from the Inspector-generated Sampling
Program) in 758 samples (26 under the Domestic Scheduled Sampling Program and 732 under the
Inspector-generated Sampling Program). Additionally, FSIS reported 22 residue violations in 2,676
samples under the Import Reinspection Sampling.
By comparison, in FY2015, there were 1,041 residue violations (17 from the domestic scheduled
sampling program and 1,024 from the Inspector-generated sampling program) in 808 samples. Note:
Multiple violative (exceeding an acceptable or tolerable level set by FDA and/or EPA) residue may be
detected in a single sample.
Domestic Scheduled Sampling
In FY 2016, under the Domestic Scheduled Sampling program, FSIS Inspection Program Personnel (IPP)
collected 7,067 residue samples (This includes 6,535 samples from U.S. Federal establishments and 532
from U.S. State plants), from which 29 violative residues were reported from 26 samples, which is less
than 1 % of the 6,445 samples collected under the Domestic Scheduled Sampling program. In FY 2015,
FSIS IPP collected 6,445 residue samples, from which 17 violative residues were reported from 12
samples (less than 1%).
During FY 2016, four carbadox, two DDT/metabolites , one doramectin, , one ivermectin, two
melengestrol acetate, seven moxidectin, one pentachlorobenzene, one permethrin, one piperonyl butoxide,
two sulfadimethoxine and seven sulfamethazine violations were reported in the Domestic Scheduled
Sampling Program.
In some cases, chemical residues were detected in samples at levels below the set tolerance levels non-
violative levels). In FY 2016, 24 samples (less than 1% of 7,067 samples collected) were considered
non-violative. By comparison, in FY 2015 the number of non-violative samples was similar, at 23 non-
violative positives (less than 1%).
Inspector-generated Sampling
In FY 2016, under the Inspector-generated sampling program, FSIS IPP screened 182,184 samples using
the Kidney Inhibition Swab (KIS™) test. Subsequently, 3,649 KIS™ test screened positive samples were
submitted to FSIS field laboratories for further analysis. For FY 2016, 883 KIS™ test residue violations
analytes were confirmed in 724 KIS™ test samples (Note: multiple residue violations may be found in
same samples.

6
For comparison, in FY2015, FSIS IPP submitted 4,022 (from 184,010 KIS™ test) samples for laboratory
confirmation. Of the 4,022 KIS™ submitted 1,017 KIS™ residure violatons were confirmed in 792
samples.
Under the Inspector-generated Sampling Program, samples from show animals, state testing program and
collected-generated were sent directly to FSIS labs, for residue Analysis. For FY 2016, under these
sampling programs Ten additional reside violative analystes were identified in eight samples submitted
under this unique sampling.
Examination of the FY 2016 Inspector-generated Sampling Program showed that the predominant
violative residues were Ceftiofur (223), Penicillin (216) and Sulfadimethoxine (76), which accounts for
25, 24 and 9% of total violative residues, respectively. In FY 2015, the top violative residues were
Ceftiofur, Penicillin, and Sulfamethazine.
In FY 2016, 728 samples with non-violative positives were observed in the Inspector-generated Sampling
Program, which was down, when compared to the 873 reported in FY 2015.
Import Reinspection Sampling
Of the 2,676 import samples analyzed, under the FY 2016 Import Reinspection Sampling Program, 22
samples had residues exceeding an acceptable or tolerable level set by FDA and/or EPA. These were from
samples originating from Nicaragua (2) and Uruguay (20). In comparison to FY2015, where seven
samples with violative residues were detected (2,922 import samples) originating from Brazil (1), Canada
(1), and Nicaragua (5).
FSIS continually strives to improve its methods for reporting of NRP data. These reports and previous
years’ residue sample results are publicly available on the FSIS website at:
http://www.fsis.usda.gov/wps/portal/fsis/topics/data-collection-and-reports/chemistry/residue-chemistry
7
Acronyms
CSI- Consumer Safety Inspector
COLLGEN – Collector-Generated Samples sent directly to the laboratory
DW – FSIS Data Warehouse
EPA- Environmental Protection Agency
FDA- Food and Drug Administration
FSIS – Food Safety and Inspection Service
HACCP – Hazard Critical Control Point
IPP – Inspection Program Personnel
KIS™ Test – Kidney Inhibition Swab Test
MRM – Multi Residue methods
ND – Non-detect
NRP- National Residue Program
OPHS – Office of Public Health Science
PHIS – Public Health Information System
PHV – Public Health Veterinarian
PPB – parts per billion
PPM – parts per million
SAT – Surveillance Advisory Team
STATE – State or Government Agency Testing
SHOW – Show Animals
U.S NRP – U.S. National Residue Program
“8888”: A numerical entry that indicate instances when chemical residues results were
detected, but were not quantitated
.

8
Introduction
The U.S. National Residue Program (NRP) for Meat, Poultry, and Egg Products, administered by the U.S.
Department of Agriculture’s (USDA), Food Safety and Inspection Service (FSIS), is an interagency
program designed to identify, rank, and analyze for chemical contaminants in meat, poultry, and egg
products. FSIS publishes the NRP Residue Sampling Plans (traditionally known as the Blue Book) each
year to provide information on the process of sampling meat, poultry, and egg products for chemical
contaminants of public health concern.
Background
F
SIS administers this regulatory program under the Federal Meat Inspection Act (FMIA)
(21 U.S.C. 601
et seq.), the Poultry Products Inspection Act (PPIA) (21 U.S.C. 453 et seq.), and the Egg Products
Inspection Act (EPIA) (21 U.S.C. 1031 et seq.). The NRP is an important component of FSIS mission to
protect the health and welfare of the consumers by regulating the meat, poultry, and egg products
produced in federally inspected establishments and to prevent the distribution in commerce of any such
products that are adulterated or misbranded.
The NRP requires the cooperation and collaboration of several agencies for its successful design and
implementation. FSIS, along with the Food and Drug Administration (FDA) and the Environmental
Protection Agency (EPA) are the primary Federal agencies managing this program. The FDA, under the
Federal Food, Drug, and Cosmetic Act (FFDCA)
, establishes tolerances for veterinary drugs and action
levels for food additives and environmental contaminants. The EPA, under the FFDCA, the Federal
insecticide, Fungicide, and Rodenticide Act (FIFRA) and the Toxic Substances Control Act (TSCA)
establishes tolerances for registered pesticides.
Title 21 Code of Federal Regulations (CFR) includes
tolerance levels established by FDA; and Title 40 CFR includes tolerance levels established by EPA.
The Surveillance Advisory Team (SAT) meets annually to evaluate chemical compounds for inclusion in
the NRP scheduled sampling plans. The SAT includes representatives from FSIS, FDA, EPA, USDA’s
Agricultural Research Service (ARS), and the USDA’s Agricultural Marketing Service (AMS), as well as
HHS’ Centers for Disease Control and Prevention (CDC). The SAT consists of experts in veterinary
medicine, toxicology, chemistry, and public health who provide professional advice, as well as
information on veterinary drug and pesticide use in animal husbandry. SAT discussions are used to
decide which compounds represent a public health concern and warrant inclusion in the NRP scheduled
sampling plans. In addition, the SAT may propose, based on professional judgment and reliable field
information, the initiation of exploratory assessments for directed sampling on a production class or
region of the country. These agencies work together to create the annual sampling plan, based on the
following: prior NRP findings of chemical residues in meat, poultry, and egg products; FDA veterinary
drug inventories completed during on-farm visits and investigation information; and pesticides and
environmental contaminants of current importance to EPA.

9
Chemical compounds analyzed in the program include approved and unapproved veterinary drugs,
pesticides, and environmental compounds. The NRP is designed to: (1) provide a structured process for
identifying and evaluating chemical compounds used in food animals; (2) analyze chemical compounds
of concern; (3) collect, analyze, and report results; and (4) identify the need for regulatory follow-up
subsequent to the identification of violative levels of chemical residues.
Actions taken on violations
F
SIS has administered the NRP by collecting and analyzing meat, poultry, and egg product samples for
specific chemical compounds at FSIS laboratories since 1967 for meat and poultry, and beginning in 1995
for egg products. A violation occurs when an FSIS laboratory detects a chemical compound level in
excess of an established tolerance or action level as well as if the residue detected has no approved
tolerance. Once the laboratory analysis is complete, FSIS enters the detailed residue violation
information into the Residue Violation Information System (RVIS), an FSIS/FDA interagency database.
FSIS provides establishment and the designated FSIS Inspection Program Personnel (IPP) with the
analysis results and also notifies the producer via certified letter. Under best practices, the establishment
also should notify the producer that an animal from that business has been identified as having a residue
violation. In addition, FSIS shares the violation data with EPA and FDA, where the latter Agency has on-
farm jurisdiction. FDA and cooperating State agencies investigate producers linked to residue violations
and, if conditions leading to residue violations are not corrected, can enforce legal action.
To notify the public and the industry of repeated residue violations by the same producer, FSIS posts a
weekly Residue Repeat Violators List
on its Web site that identifies producers with more than one
violation on a rolling 12-month period. In addition, the list provides helpful information to the AMS-
School Lunch Program purchase clearance processors and producers who are working to avoid illegal
levels of residues, serves as a deterrent for violators, and enables FSIS and FDA to make better use of
resources (
list for processors and producers). Because FSIS updates are posted weekly, FDA may not
have investigated each violation at the time of publication.
FSIS Laboratory Analytical Methods
In January 1997, FSIS implemented the Hazard Analysis and Critical Control Point (HACCP) inspection
system in all federally inspected establishments. The HACCP regulation (HACCP GPO CFR
) requires
FSIS-inspected slaughter and processing establishments to identify all food safety hazards (including drug
residues, chemical contaminants, and pesticides) that are reasonably likely to occur before, during, and
after the food animal or product enters the slaughter establishment. The regulation also requires
establishments to identify preventive measures to control these hazards. FSIS takes regulatory action
against establishments that do not have an effective chemical residue control program in place.
Minimizing food safety hazards from farm-to-fork protects consumers from the public health risks
associated with chemical contaminants in food.
With greater public concern about the risks of chemical contaminants, focus has increased on
strengthening the identification, prioritization, and testing for chemical hazards in meat, poultry, and egg
products in the United States. The sampling plan for residues in FSIS-regulated products includes
strengthening the focus of public health-based sampling. This approach includes broader screens for
veterinary drugs, pesticides, and heavy metals, as well as conducting more analyses per sample.

10
FSIS uses analytical methods to detect, identify, and quantify residues that may be present in meat,
poultry, and processed egg products. The Agency utilizes these methods for monitoring and for
surveillance activities to determine product adulteration and for evaluations of human health risk. The
Agency uses available methodologies to take appropriate regulatory action against adulterated products in
a manner consistent with the reliability of the analytical data. The
FSIS Analytical Chemistry Laboratory
Guidebook lists the analytical methods used by the agency.
Figure 1. National Residue Program: The figure illustrates the intricate steps of the NRP. The NRP
begins with interagency planning (Blue Book) of sampling program, which is followed by collection and
analysis of samples reported (Red Book).

11
Overview of the Sampling Plans
The United States Government Fiscal Year (FY) runs from October 1 through September 30. To match
this, since 2012, FSIS switched from implementing the NRP on a Calendar Year (CY) to a FY basis.
This change allows the program to run concurrently with the Federal budget cycle.
The NRP consists of three separate, but interrelated, chemical residue testing programs: scheduled
sampling (Tier 1), targeted sampling at the production or compound class level (Tier 2), and targeted
sampling at the herd/flock or compound class level (Tier 3). This basic structure has been in existence
since 1967. These testing programs provide data for FSIS to detect chemical residues of public health
concern and have been modified annually in response to emerging chemical residue concerns and
improved testing methodologies.
The 2016 NRP Residue Sampling Plan focuses on chemical residues in domestic meat, poultry, and egg
products and the import reinspection of meat, poultry, and egg products. The domestic sampling plan
includes scheduled sampling and inspector-generated sampling. The import reinspection sampling plan
encompasses normal sampling, increased sampling, and intensified sampling. Directive 10,800.1, Rev 1
provides further detail on those sampling procedures.
Domestic Sampling Plan
1. Tier 1
The Tier 1 sampling plan is the scheduled sampling of specified slaughter subclasses at the time of
slaughter, after they have passed antemortem inspection. Carcasses are randomly selected for sampling.
The number of samples scheduled each year is based on the probability of detecting at least one violation
(Appendix II). Data collected from Tier 1 sampling serves as a baseline level for chemical residue
exposure. Sampling tasks are assigned each month through the Public Health Information System
(PHIS). The sampling task provides information to the Inspection Program Personnel (IPP) on when to
collect the sample (collection window) and which production class to sample. The establishment holds or
controls livestock carcasses selected for testing pending the results of analysis. For directed testing of
poultry, the IPP recommends to the establishment that the establishment holds the specific poultry
carcasses selected for residue testing pending the analysis results.
Tier 1 sampling results also can be used to identify producers or other entities marketing animals with
violative levels of residues. Thus, the Tier 1 sampling plan not only gathers information, but also assists
in deterring practices that lead to violative residues.
In 2016, the Tier 1 sampling plan consisted of random samples collected from each of the following
production classes: beef cows, bob veal, dairy cows, steers/ heifers, market hogs, sows, young chickens,
and young turkeys. These production classes represent 95 percent of domestic meat and poultry
consumption.

12
2. Tier 2
a. Inspector-Generated Sampling
FSIS inspection program personnel (IPP) conduct inspector-generated sampling when they suspect that
animals may have violative levels of chemical residues. Currently, inspector-generated sampling targets
individual suspect animals, suspect populations of animals, and animals condemned for specific
pathologies listed in FSIS Directive 10,800.1, Rev 1
. When Public Health Veterinarians (PHVs) detect
evidence of a disease that may have been treated or suspect the administration of a drug, they retain the
carcass and analyze samples from those carcasses using an in-plant method to screen for the presence of
chemical residues. If the in-plant test is negative for antimicrobial residues included in the screen, the
carcass is released to the establishment. If there are screen positive results, the carcass is held pending the
results of laboratory testing. The PHV condemns carcasses of animals found to contain violative levels of
residues in the muscle or if an unapproved drug is detected in any tissue.
In 2016, IPP completed in-plant residue screens using the Kidney Inhibition Swab test (KIS™ test). The
screen positive samples are submitted to the FSIS Midwestern Laboratory and analyzed by the laboratory
to identify, quantify and confirm the contaminants.
i. Sampling of Individual Suspect Animals
Under the direction of the PHV, IPP are to conduct a KIS™ test on any carcass that based on herd history
or ante-mortem or post-mortem findings inspection findings may contain a violative drug residue. IPP are
to follow the instructions provided in Directive 10,800.1, Rev 1
, chapter three for circumstances
warranting a KIS ™ test and Chapter Four for performing KIS™ tests and documenting the task in PHIS.
The PHV selects a carcass for sampling based on the criteria outlined in
FSIS Directive 10,800.1, Rev 1
(i.e., animal with disease signs and symptoms, producer history, or as a follow-up to results from random
scheduled sampling). Usually, the sample is screened in the plant by the IPP and the screen-result verified
when necessary by a PHV. Other samples are sent directly to the laboratory for analysis. For example, if
the IPP suspects the misuse of a veterinary drug in an animal, she/he can perform the relevant in-plant
screening analysis. If the result of a screening analysis is positive, the carcass is held (if it is not already
condemned for other pathology or conditions that would make it unfit for human consumption), and the
liver, kidney, and muscle samples from the carcass are then sent to an FSIS laboratory for analysis and
confirmation.
ii. Sampling of Suspect Animal Populations
Sampling for suspect animal populations is directed by an FSIS regulation (9 CFR 310.21) and
Directive
10,800.1, Rev 1. This is outlined for healthy appearing bob veal calves and show animals.
b. Targeted Sampling
FSIS implements targeted sampling plans (exploratory assessments) in response to information (obtained
by FDA and EPA and provided to FSIS) about misuse of animal drugs and/or exposure to environmental
chemicals, as well as in response to Tier 1 analytical results. The duration of these sampling plans vary
based on the situation. FSIS may conduct studies to develop information on the frequency and
concentration at which some residues like trace metals and industrial components may be inadvertently
present in animals. These sampling plans could be designed to distinguish components of meat, poultry
and egg products in which residue problems exist, to measure the extent of problems, and to evaluate the
impact of actions taken to reduce the occurrence of residues in the food animal population.
Sampling tasks are assigned through PHIS. The sampling task provides instructions to the IPP on when
to collect the sample (collection window) and which slaughter production class to collect from. The
establishment holds or controls livestock carcasses selected for testing pending the test results. For

13
directed residue testing of poultry, the IPP recommends to the establishment that the establishments hold
the specific poultry carcasses selected for residue testing pending the test results.
In 2016, targeted sampling included old breeder turkeys, and sheep, goats.
3. Tier 3
The Tier 3 sampling plan is similar in structure to the targeted sampling (exploratory assessment)
program in Tier 2, with the exception that Tier 3 will encompass targeted testing at a herd or flock level.
A targeted testing program designed for livestock or flocks originating from the same farm or geographic
region may be necessary on occasion to determine the level of exposure to a chemical or chemicals. For
instance, producers may administer some veterinary drugs to a herd or a flock (for example, growth
promotants or antibiotics given in the feed) in a way that involves misuse. In addition, livestock and birds
may be exposed unintentionally to an environmental contaminant. Therefore, a targeted testing program
designed for livestock or flocks originating from the same farm or region may be necessary on occasion
to determine the level of a chemical or chemicals to which the livestock or the birds in the flock have
been exposed. Tier 3 will provide a vehicle for developing information that will support future policy
development within the NRP.
In FY 2016, no Tier 3 sampling was performed.
Import Reinspection Sampling Plan
Imported meat, poultry, and egg products are sampled through the port-of-entry Import Reinspection
Sampling Plan, a chemical residue monitoring program conducted to verify the equivalence of inspection
systems in exporting countries to the United States standards. All imported products are subject to
reinspection, and one or more types of inspection (TOI) are conducted on every lot
2
of product before it
enters the U. S. Chemical residue sampling is included in the reinspection of imported products. The
following three levels of chemical residue reinspection include:
• normal sampling: random sampling from a lot;
• increased sampling: above-normal sampling resulting from an Agency management decision; and
• intensified sampling: additional samples taken when a previous sample for a TOI that failed to
meet U. S. requirements.
The data obtained from laboratory analyses are entered into PHIS, an FSIS database designed to generate
reinspection assignments, receive and store results, and compile histories for the performance of foreign
establishments certified by the inspection system in the exporting country.
The import reinspection sampling program is structured using the Tier 1 and Tier 2 criteria used to
develop the domestic plan. In FY2016, FSIS collected approximately 2676 import samples.
2
An import lot is a group of products defined statistically and/or scientifically by production segments and certified from one
country, one establishment. A lot consists entirely of the same species, process category, and product standard of identity (sub-
category). A single lot can contain shipping cartons with varying sizes of immediate containers.

14
Policy and procedures for holding or controlling product under NRP
As of February 2013, the Agency requires official establishments and importers of record to hold or
maintain control of lots of product tested for adulterants until acceptable results become available. FSIS
stated that the policy would apply to livestock carcasses subject to FSIS testing for residue on domestic
products. FSIS explained that it will not hold poultry carcasses pending test results for residues due to
historically low residue problems and large lot size. This was outlined in a published
Federal Register
Notice 76 FRN 19955.
The Hold and Test policy also applies to normal and increased import reinspection sampling.
Additionally, for intensified import sampling, the lot must be retained pending laboratory results.
15
Domestic Scheduled Sampling Program
This section reports the summary results from the FSIS Domestic Scheduled Sampling Plan. The
summary results are associated with specific Animal Class. All data reported in the following tables were
collected from the FSIS Data Warehouse and PHIS databases.
Table 1 identifies the animal classes and methods/chemical classes which are in the 2016 NRP
Table 2 summarizes the number of Domestic Scheduled samples and Inspector-generated samples tested
by animal class.
Table 3 summarizes the number of residue Domestic Scheduled samples analyzed by animal class,
including summary results.
Table 4 summarizes the number of residue Domestic Scheduled samples tested per chemical method by
animal class.
Table 5 summarizes Domestic Scheduled Sampling -number of chemical analyses tested per chemical
method by animal class.
Table 6 summarizes domestic scheduled sampling violation results by animal class.
Note: Residue detected results with “8888” indicate instances when residues were detected, but were not
quantitated.

16
Summary of Domestic Residue Sampling Program
Table 1. FY 2016 Tier I and II List of Animal Class by Method/Chemical Class (Analyses Performed)
Animal
Category
Animal Class
Chemical Class
Oct 2015- Sep 2016
Aminoglycosides Arsenic Avermectins
βeta-
Agonists
Carbadox Hormones Metals MRM Nitrofurans Pesticides
Bovine
Beef Cows
√ √
√ √
--
√ √ √
--
√
Bob Veal
√ √ √ √
--
√ √ √
--
√
Dairy Cows
√ √ √ √
--
√ √ √
--
√
Heifers
√ √ √ √
--
√ √ √
--
√
Steers
√ √ √ √
--
√ √ √
--
√
Porcine
Market Swine
√ √ √ √
-- --
√ √
--
--
Roaster Swine
√ √ √
- √
--
√ √
--
√
Sows
√ √ √
√
--
--
√ √
--
√
Poultry
Mature Turkeys
√ √
-- -- -- --
√ -
√
--
Young Chickens
√ √
-- -- -- --
√ √ √
√
Young Turkeys
√ √
-- -- -- --
√ √ √
√
Minor Species
Goats
√ √ √
-- -- -- --
√
-- --
Sheep
√ √ √
-- -- -- --
√
-- --

17
Table 2. FY 2016 Number of Scheduled Residue Samples Tested, by Animal Class
Animal
Category
Animal Class
Domestic Scheduled Sampling
Inspector-generated Sampling
Tier-2 Suspect Animals
Tier-1 & Tier- 2*
U.S. Federal
Plants
Tier-1
U.S. State
Plants
KIS™ Test
COLLEGEN/
SHOW/STATE *
Bovine
Beef Cows
670
60
15,936
12
Bob Veal
574
--
23,333
4
Bulls
--
--
1,618
2
Dairy Cows
720
19
99,660
23
Formula-Fed Veal
--
--
640
--
Heavy Calves
--
--
426
--
Heifers
397
129
2,537
6
Non-Formula-Fed Veal
--
--
161
--
Steers
366
145
8,705
16
Porcine
Boars/Stags
--
--
99
--
Market Swine
684
116
18,754
46
Roaster Swine
281
--
1,527
--
Sows
733
36
6,461
3
Poultry
Mature Turkeys**
93
--
--
--
Young Chickens
742
18
--
--
Young Turkeys
648
9
--
--
Goats**
337
--
618
7
Minor
Species
Lambs**
--
--
1,224
10
Sheep**
290
--
485
--
Total 6,535 532 182,184* 129
* An additional 129 inspector-generated samples were collected and sent to FSIS labs for analysis. These
samples are associated with project codes: 75 COLLGEN, 42 SHOW, and 12 STATE, samples.
** Animal Classes associated with NRP Tier 2 domestic sampling

18
Table 3. FY 2016 NRP Domestic Scheduled Samples Analyzed by Animal Class –
and Summary Results
Animal
Category
Animal Class
Number
of Non-
Detect
Samples
Number
of Non-
Violative
Positives
Samples
Number of
Violative
Samples
Total
Samples
Bovine
Beef Cows
727
2
1
730
Bob Veal
568 3
3
574
Dairy Cows
736
--
3
739
Heifers
519 5
2
526
Steers
507 4
--
511
Porcine
Market Swine
798 2
--
800
Roaster Swine
271 4
6
281
Sows
765 3
1
769
Poultry
Mature Turkeys
93
--
--
93
Young Chickens
759 1
--
760
Young Turkeys
657
-- --
657
Minor Species
Goats
330
--
7
337
Sheep
287
--
3
290
Total
7017
24 26
7,067
Note: The results include Tier 1 and Tier 2 animal classes
Data Source: FSIS Data Warehouse and PHIS databases.

19
Table 4. FY2016 NRP Residue Scheduled Samples -Number of Residue Samples Tested Per Chemical Method by
Animal Class
Animal Class
(# Samples Collected)
Number of Samples per Chemical Method
Aminoglycosides Arsenic Avermectins
βeta-Agonists
Carbadox Hormones Metals MRM Nitrofurans Pesticides
Beef Cows (730)
725 397 392 289
--
357 114 730
--
286 (1)
Bob Veal (574)
571 326 323 (1) 216
--
294 118 574 (2)
--
211
Dairy Cows (739)
737 395 392 302
--
348 112 739 (2)
--
304 (1)
Heifers (526)
524 313 310 180
--
294 (2) 114 526
--
177
Steers (511)
510 306 303 175
--
276 107 511
--
175
Market Swine (800)
798 447 442 150 2
--
127 799
--
333
Roaster Swine (281)
280 65 64
--
215 (4)
--
17 281 (2)
-- --
Sows (769)
764 427 421 135 - 1 111 769
--
290 (1)
Mature Turkeys (93)
1 1
-- -- -- --
93 1
-- --
Young Chickens (760)
759 408
-- -- -- --
155 760 340 316
Young Turkeys (657)
656 371 1
-- -- --
154 657 275 141
Goats (337)
260 195 198 (7) 1
-- -- --
337
--
141
Mature Sheep (290)
200 155 153 (1) 1
-- -- --
290
--
131 (2)
Total (7,067) 6,785 3,806 2,999 1,449 217 1,570 1,222 6,974 615 2,505
Note: Number of violative samples (in parenthesis)
Data Source: FSIS Data Warehouse and PHIS databases.

20
Table 5. FY 2016 NRP Residue Scheduled Samples - Number of Chemical Analytes Tested Per Chemical Method by
Animal Class
Animal Class (#
Samples Collected)
Number of Chemical Analytes per Chemical Method
Aminoglycosides Arsenic Avermectins βeta-Agonists
Carbadox
Hormones Metals MRM Nitrofurans Pesticides Total
Beef Cows (730)
7,259 397 1,958 1,732
--
1,785 1,198 58,305
--
24,417 97,051
Bob Veal (574)
5,728 326 1,612 1,296
--
1,470 1,361 46,094
--
17,751 75,638
Dairy Cows (739)
7,379 395 1,960 1,808
--
1,740 1,235 59,252
--
25,724 99,493
Heifers (526)
5,249 313 1,550 1,061
--
1,468 1,397 42,138
--
14,832 68,008
Steers (511)
5,109 306 1,513 1,033
--
1,380 1,262 41,071
--
14,647 66,321
Market Swine (800)
7,999 447 2,205 896 2
--
1,480 69,240
--
28,134 110,403
Roaster Swine (281)
2,836 65 320
--
215
--
298 28,137
--
--
31,871
Sows (769)
7,658 427 2,102 805 - 5 1,081 67,045
--
24,516 103,639
Mature Turkeys (93)
10 1
--
--
--
--
1,008 93
--
--
1,112
Young Chickens (760)
7,599 408
--
--
--
--
1,743 64,022 1,700 26,716 102,188
Young Turkeys (657)
6,569 371 5
--
--
--
1,925 54,081 1,374 21,110 85,435
Goats (337)
2,600 195 984 6
--
--
--
28,061
--
11,826 43,672
Mature Sheep (290)
2,000 155 762 2
--
--
--
23,260
--
11,115 37,294
Total (7,067)
67,995 3,806 14,971 8,639 217 7,848 13,988 580,799 3,074 220,788 922,125
Note: Multiple analytes may be associated with the same sample. Not all samples are tested for all chemical method. Number of
samples per chemical method is indicated in Table 4
Data Source: FSIS Data Warehouse and PHIS databases.

21
Table 6. FY 2016 Domestic Scheduled Sampling Plan Violations
Animal Tissue Compound Concentration Units
Tolerance
Level
Value
Authority
(CFR
Citation)
Beef Cow Muscle Piperonyl Butoxide 0.162 ppm 0.1 40 CFR 180.127
Bob Veal Muscle Sulfamethazine 22.500 ppm 0.1 21 CFR 556.670
Bob Veal
Muscle Sulfamethazine 0.190 ppm 0.1 21 CFR 556.670
Liver Sulfamethazine 0.304 ppm 0.1 21 CFR 556.670
Bob Veal Muscle Moxidectin 16.1 ppb 0 21 CFR 556.426
Dairy Cow Liver Sulfadimethoxine 0.114 ppm 0.1 21 CFR 556.640
Dairy Cow Liver Sulfadimethoxine 1.064 ppm 0.1 21 CFR 556.640
Dairy Cow Muscle
Permethrin
(Cis and Trans)
0.213 ppm 0.1 40 CFR 180.378
Heifer Muscle Melengestrol Acetate 2.2 ppb None 21 CFR 556.380
Heifer Muscle Melengestrol Acetate 1.3 ppb None 21 CFR 556.380
Roaster Swine
Liver Sulfamethazine 0.702 ppm 0.1 21 CFR 556.670
Muscle Sulfamethazine 0.237 ppm 0.1 21 CFR 556.670
Roaster Swine Liver Carbadox 78.035 ppb 30 21 CFR 556.100
Roaster Swine Liver Carbadox 131.001 ppb 30 21 CFR 556.100
Roaster Swine Liver Carbadox 31.406 ppb 30 21 CFR 556.100
Roaster Swine Liver Carbadox 68.511 ppb 30 21 CFR 556.100
Roaster Swine
Muscle Sulfamethazine 0.117 ppm 0.1 21 CFR 556.670
Liver Sulfamethazine 0.227 ppm 0.1 21 CFR 556.670
Sow Muscle DDT and Metabolites ***
Goat Muscle Moxidectin 77.05 ppb
Not
Approved
21 CFR 556.426
Goat Muscle Moxidectin 29.45 ppb
Not
Approved
21 CFR 556.426
Goat Muscle Moxidectin 48.4 ppb
Not
Approved
21 CFR 556.426
Goat Muscle Moxidectin 30.9 ppb
Not
Approved
21 CFR 556.426

22
Table 6. FY 2016 Domestic Scheduled Sampling Plan Violations – Federal Plants
Note:
****: Violative residue results were residue were detected but not quantified
Not Approved- Residue detected is not approved per species
Data Source: FSIS Data Warehouse and PHIS databases.
Animal Tissue Compound Concentration Units
Tolerance
Level
Value
Authority
(CFR
Citation)
Goat Muscle Moxidectin 56.8 ppb
Not
Approved
21 CFR 556.426
Goat Muscle Ivermectin 72.45 ppb
Not
Approved
21 CFR 556.344
Goat Liver Moxidectin 224 ppb
Not
Approved
21 CFR 556.426
Sheep Muscle DDT and Metabolites ***
Sheep Muscle Pentachlorobenzene ***
Sheep Muscle Doramectin 168.5 ppb 30 21 CFR 556.225
23
Summary of Domestic Inspector -Generated Sampling Program
PHVs, and CSIs under the guidance of a PHV, conduct Inspector-generated residue sampling when an
animal is suspected to have undergone drug treatment and may possibly contains violative levels of
chemical residues. The PHVs and CSIs also are encouraged to collect samples for residue testing at the
FSIS labs when a chemical contamination is suspected. Samples are screened using the KIS™ test. If
KIS™ test kits are not available; the PHV submits the sample to the FSIS laboratory for testing.
Table
7 summarizes the total number in-plants screens tests using the KIS™ test, which includes the
number of in-plants screens with negative results, number of positive screens sent to FSIS labs for
conformation, and the number of carcasses with violations for each animal class.
Table
8 summarizes the total number of samples analyzed and the number of carcasses with violations
for each animal class under additional inspector-generated program projects such as COLLGEN, SHOW,
and STATE.
Table
9 summarize the results for specific chemical compounds that were detected (violative) within
inspector-generated sampling project (including the KIS™) across animal class.
Table
10 summarize the results for specific chemical compounds that were detected (non-violative)
within inspector-generated sampling project (including the KIS™) across animal class.
Note: Data in this document were obtained from the FSIS Data Warehouse and PHIS databases.

24
Table 7. FY 2016 Tier II Inspector Generated Sampling (KIS
TM
) Test
Animal
Category
Animal Class
KIS ™ Test
Total Number of
In-plant
Samples
Number of In-
plant
Negative
Samples
Number of In-plant
Positive
Samples
Number of
Samples With
Confirmed Lab
Violations
Bovine
Beef Cows
15,936
15,582
354
51
Bob Veal
23,333
22,961
372
103
Bulls
1,618
1,565
53
13
Dairy Cows
99,660
97,384
2276
480
Formula-Fed Veal
640
627
13
1
Heavy Calves
426
404
22
9
Heifers
2,537
2,486
51
5
Non-Formula-Fed Veal
161
157
4
0
Steers
8,705
8,530
175
33
Porcine
Boars/Stags
99
98
1
0
Market Swine
18,754
18,579
175
4
Roaster Swine
1,527
1,507
20
1
Sows
6,461
6,354
107
21
Minor
Species
Goats
618
614
4
0
Lambs
1,224
1,212
12
2
Sheep
485
475
10
1
Total
182,184
178,535
3,649
** 724
** 883 KIS ™ test violative analytes in 724 lab confirmed KIS ™ test violative samples. Multiple violative analytes in different
tissue types may be associated with a single sample (Carcass).
Data Source: FSIS Data Warehouse and PHIS databases.

25
Table 8. FY 2016 Tier II Inspector-Generated Sampling (COLLGEN/ STATE/ SHOW) Projects
Animal
Category
Animal Class
COLLGEN SHOW STATE
Number of
Samples
Number of
Samples With
Confirmed
Lab Violations
Number
of
Samples
Number of
Samples With
Confirmed
Lab Violations
Number of
Samples
Number of
Samples With
Confirmed Lab
Violations
Bovine
Beef Cows 7
--
--
--
5
1
Bob Veal 4
2
--
--
--
Bulls
1
--
--
--
1
--
Dairy Cows 23
2
--
--
--
--
Formula-Fed Veal
--
--
--
--
--
--
Heavy Calves
--
--
--
--
--
--
Heifers 5
1
--
--
1
--
Non-Formula-Fed Veal
-- --
--
--
--
--
Steers
4
--
11
--
1
--
Porcine
Boars/Stags
--
--
--
--
--
--
Market Swine 22
--
21
1
3
--
Roaster Swine
--
--
--
--
--
--
Sows 3
--
--
--
--
--
Minor Species
Goats 3
--
4
--
--
--
Lambs 3
--
6
--
1
1
Sheep
--
--
--
--
--
--
Total 75 5 42 1 12 2
Note: Results include two violative residues from two dairy cow (penicillin, florfenicol and sulfamethazine), two bob veal (penicillin and
sulfamethazine), a beef cow (desfuroylceftiofur) and one heifer (sulfadimethoxine), one market swine (sulfamethazine) and a lamb (penicillin).
Data Source: FSIS Data Warehouse and PHIS databases
.

26
Table 9. FY 2016 Number of Residue Violations results in Inspector Generated Sampling by Chemical Residue and
Animal Class ( include
KIS ™ test, COLLGEN/ STATE/ SHOW project codes)
Note: Multiple violative analytes in different tissue types may be associated with a single sample (carcass).
Data Source: FSIS Data Warehouse and PHIS databases.
Chemical Residue
Beef Cows
Bob Veal
Bulls
Dairy Cow
Formula
Fed Veal
Heavy Calves
Heifer
Steers
Market
Swine
Roaster Swine
Sows
Lamb
Sheep
Total
Amikacin -- -- -- 1 -- -- -- -- -- -- -- -- --
1
Ampicillin -- -- -- 28 -- -- -- -- -- -- -- -- --
28
Cefazolin
-- -- --
1
-- -- -- -- -- -- -- -- --
1
Ciprofloxacin -- -- 1 1 -- 1 -- 1 -- -- 1 -- --
5
Desethylene Ciprofloxacin -- 1 -- -- -- -- -- -- -- -- -- -- --
1
Desfuroylceftiofur 13 7 3 192 -- -- 2 6 -- -- -- -- --
223
Dihydrostreptomycin -- 2 -- 3 -- -- -- -- -- -- -- -- --
5
Enrofloxacin -- 1 -- -- -- -- -- -- -- -- -- -- --
1
Florfenicol 15 2 8 11 -- 6 -- 7 -- -- -- -- --
49
Flunixin
6
6
1
49
--
1
2
3
--
--
2
1
71
Gentamycin Sulfate 4 -- -- 4 -- -- -- 3 -- -- -- -- 1
12
Ketoprofen -- -- -- 2 -- -- -- -- -- -- -- -- --
2
Lincomycin -- -- -- 5 -- -- -- -- -- -- -- -- --
5
Meloxicam
-- --
1 3
-- -- -- -- -- -- -- -- --
4
Moxidectin
1
-- --
-- -- -- -- -- -- -- -- -- --
1

27
Table 9. FY 2016 Number of Residue Violations results in Inspector Generated Sampling by Chemical Residue and
Animal Class ( include
KIS ™ test, COLLGEN/ STATE/ SHOW project codes) (cont.)
Note: Multiple violative analytes in different tissue types may be associated with a single sample (carcass)
Data Source: FSIS Data Warehouse and PHIS databases.
Chemical Residue
Beef Cows
Bob Veal
Bulls
Dairy Cow
Formula
Fed Veal
Heavy Calves
Heifer
Steers
Market
Swine
Roaster Swine
Sows
Lamb
Sheep
Total
Neomycin
--
57
--
2
--
2
--
3
--
--
-- -- --
64
Oxyphenylbutazone
--
--
--
--
--
--
--
1
--
--
-- -- --
1
Oxytetracycline
2
--
3
8
--
--
--
--
--
--
-- -- --
13
Penicillin
18
13
1
153
1
1
--
9
1
1
16 2 --
216
Phenylbutazone
1
--
--
--
--
--
--
--
--
--
--
--
--
1
Ractopamine
-- -- -- -- -- -- -- 1 -- -- -- -- --
1
Sulfadiazine
-- 2 -- -- -- -- -- -- -- -- -- -- --
2
Sulfadimethoxine
3
5
--
67
--
--
1
--
--
--
-- -- --
76
Sulfadoxine
-- -- 1 4 -- -- -- -- -- -- 1 -- --
6
Sulfamethazine
5
16
2
27
--
4
--
8
5
--
1
--
--
68
Sulfamethoxazole
--
5
--
1
-- -- -- -- -- -- -- -- --
6
Sulfamethoxypyridazine
-- -- -- 1 -- -- -- -- -- -- -- -- --
1
Tetracycline
--
--
--
1
--
--
--
--
--
--
--
--
--
1
Tilmicosin
6
3
1
8
--
2
1
3
--
--
--
--
--
24
Tylosin
1
--
--
2
--
--
--
1
--
--
--
--
--
4
Total
75 120 22 574 1 17 6 46 6 1 21 3 1 893

28
Table 10. FY 2016 Number of Non-Violative results in Inspector Generated Sampling by Chemical Residue and
Animal Class ( include
KIS ™ test, COLLGEN/ STATE/ SHOW project codes)
Note: Multiple violative analytes in different tissue types may be associated with a single sample (Carcass).
Data Source: FSIS Data Warehouse and PHIS databases.
Chemical Residue
Beef Cows
Bob Veal
Bulls
Dairy Cows
Formula -
Fed Veal
Heavy
Calves
Heifers
Non
Formula -
Fed Veal
Steer
Boar/Stag
Market
Swine
Roaster
Swine
Sows
Lambs
Total
Chlortetracycline 1 -- 1 -- -- -- -- 1 -- -- 2 -- -- --
5
Desfuroylceftiofur -- -- -- 18 -- -- -- -- 2 -- -- -- -- --
20
Dihydro
Streptomycin
-- -- -- 2 -- -- -- -- -- -- -- -- -- --
2
Dihydrostreptomycin -- -- -- 1 -- -- -- -- -- -- -- -- -- --
1
Enrofloxacin 1 -- 1 1 -- 1 -- -- 2 -- 2 -- 2 --
10
Eprinomectin 3 -- -- 14 -- -- 2 -- 3 -- -- -- -- --
22
Fenbendazole -- -- -- 4 -- -- -- -- -- -- -- -- -- --
4
Fenbendazole sulfone 1 -- -- 2 -- -- -- -- -- -- -- -- -- --
3
Florfenicol 3 -- 1 6 -- -- -- -- 3 -- -- -- -- --
13
Flunixin 3 -- 1 40 -- 1 1 -- 1 -- -- -- 3 --
50
Gamithromycin 2 -- -- 6 -- 1 1 -- 2 -- -- -- -- --
12
Ivermectin -- -- 1 -- -- -- -- -- -- -- -- -- -- --
1
Lincomycin -- -- -- -- -- -- -- -- -- -- 15 -- 5 --
20

29
Table 10. FY 2016 Number of Non-Violative results in Inspector Generated Sampling by Chemical Residue and
Animal Class ( include
KIS ™ test, COLLGEN/ STATE/ SHOW project codes) (cont.)
Note: Multiple violative analytes in different tissue types may be associated with a single sample (Carcass).
Data Source: FSIS Data Warehouse and PHIS databases.
Chemical Residue
Beef Cows
Bob Veal
Bulls
Dairy Cows
Formula -
Fed Veal
Heavy
Calves
Heifers
Non
Formula -
Fed Veal
Steer
Boar/Stag
Market
Swine
Roaster
Swine
Sows
Lambs
Total
Moxidectin 2 -- 1 -- -- -- -- -- -- -- -- -- -- --
3
Neomycin
3
25
--
8
--
3
--
1
4
--
--
--
--
--
44
Oxytetracycline 38 29 9 61 -- 4 1 -- 7 -- -- -- 3 1
153
Penicillin 6 2 2 71 1 -- -- -- 3 -- -- -- -- --
85
Pirlimycin -- 1 -- 10 -- -- -- -- -- -- -- -- -- --
11
Ractopamine -- -- -- -- -- -- -- -- 1 -- 5 -- -- --
6
Spectinomycin 4 6 1 19 -- 2 1 -- 1 -- -- -- -- --
34
Sulfadimethoxine 2 -- -- 10 -- -- 1 -- -- -- -- -- -- --
13
Sulfamethazine -- 2 -- 3 -- 1 -- 1 2 -- -- 1 -- --
10
Tetracycline 3 2 -- 30 -- -- -- -- -- -- -- -- -- --
35
Tildipirosin 3 -- -- 1 -- 1 2 -- 4 -- -- -- -- --
11
Tilmicosin 3 -- 1 1 -- -- -- -- -- -- -- 1 3 --
9
Tulathromycin 29 4 7 36 -- 2 16 -- 54 1 2 -- -- --
151
Total 107 71 26 344 1 16 25 3 89 1 26 2 16 1 728

30
Import Residue Reinspection Sampling Program
In FY2016, FSIS collected 2,676 import samples and analyzed for 169,490 residue analytes from 25
export countries. Twenty Two violations were detected (20 from uruguaw, and two from Nicaragua). For
more information, refer to the list of tables below.
Table 11 summarizes the – import number of residue samples tested per chemical method by Production
Class and Product Type
Table 12 summarizes the number of import residue samples by inspection level, per exporting country
and production type
Table 13 summarizes the number of import residue samples analyzed, by exporting country and
Production Type
Table 14 summarizes the number of import residue samples analyzed, number of chemical analyates
tested per exporting country and production type
Table 15 summarize number of samples and chemical residues under the import residue sample program,
by exporting country
Table 16 summarize import residue sample program (Non-Violative and Violative) results, by exporting
country chemical residues and production class
information for countries wanting to import to the United States can be found at:
Importing products to the United States
Information on US products eligible for export can be found at:
Export Library
31
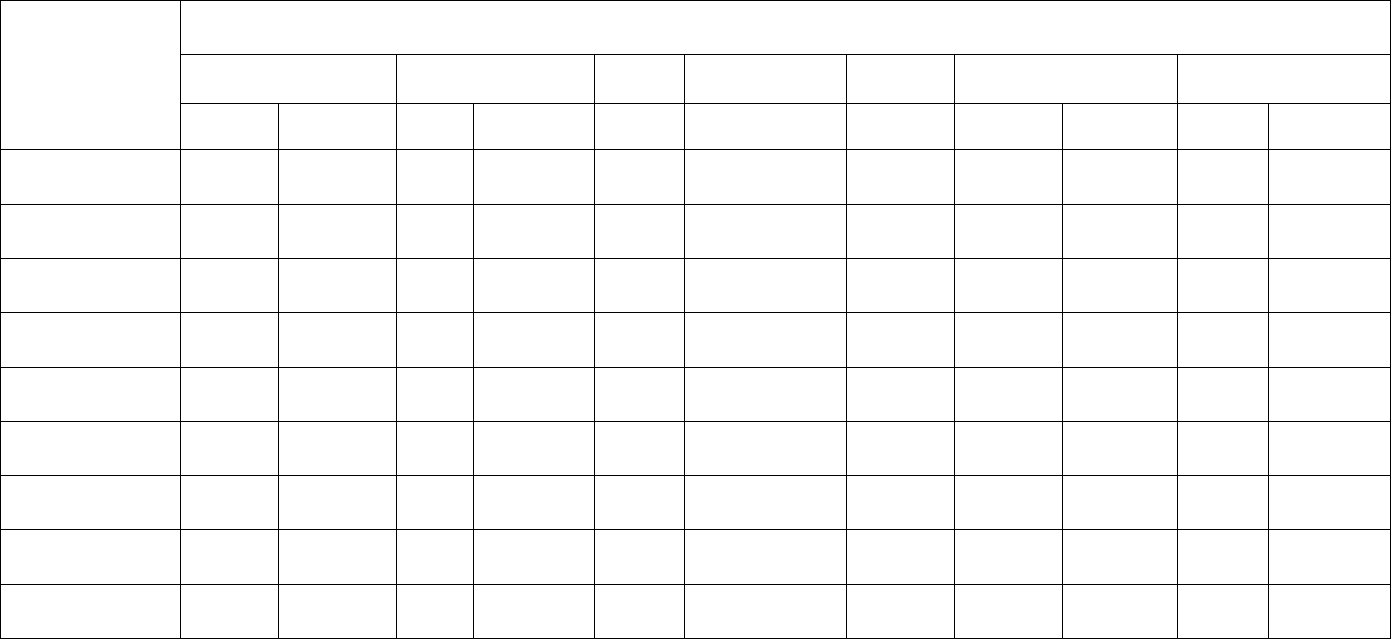
Table 11. FY 2016 NRP Import Residue Samples - Number of Residue Samples Tested Per Chemical Method by
Production Class and Product Type
Methods
Number of Samples Tested
Beef Pork Veal Lamb/Mutton Goat Chicken Turkey
Fresh Processed Fresh Processed Fresh Fresh Fresh Fresh Processed Fresh Processed
MRM
252
--
115
--
68 51 22 106
--
60
--
Aminoglycoside
251
--
198
--
68 50 20 107
--
59
--
Pesticides
719
--
128
--
45 50 37 57
--
40
--
Hormones
166
-- -- -- -- -- -- -- -- -- --
βeta-Agonists
110
--
91
--
39 5 1 1
-- -- --
Avermectins
125 117 100 51 25 48 21
-- --
1
--
Arsenic
127 115 100 51 25 48 21 57 31 23 61
Metals
71 18 57 41 24
-- --
41 11 18 24
Sulfonamides --
32 --- 46 1
-- --
1
-- --
24
Data Source: FSIS Data Warehouse and PHIS databases.

32
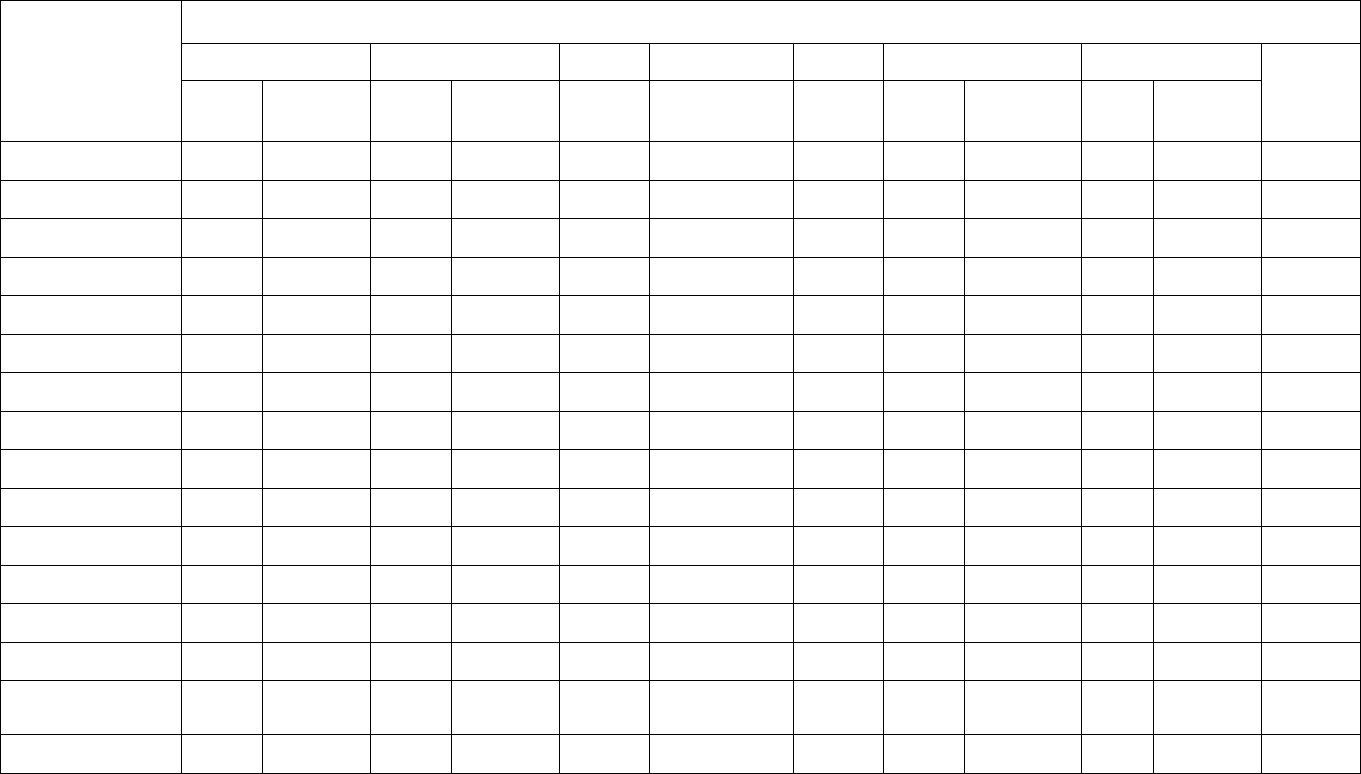
Table 12. FY 2016 Number of Import Residue Samples by Inspection Level, per
Exporting Country and Production Type
Country
Normal Increased Intensified
Total
Fresh Processed Processed Fresh Processed
Australia
160 8
--
--
--
168
Brazil
64 63
--
--
--
127
Canada
517 141
--
--
6
664
Chile
142 10
--
--
--
152
Costa Rica
12
--
--
--
--
12
Denmark
24 9
--
--
--
33
Finland
3
--
--
--
--
3
France
--
2
--
--
--
2
Germany
--
12
--
--
--
12
Iceland
48
--
--
--
--
48
Ireland
103
--
--
--
--
103
Israel
--
85
--
--
--
85
Italy
--
11
--
--
--
11
Japan
37
--
--
--
--
37
Korea, Republic Of
--
1
--
--
--
1
Lithuania
5 30
--
--
--
35
Mexico
173 20
--
--
2
195
Netherlands
16
--
--
--
--
16
New Zealand
99 12
--
--
--
111
Nicaragua
85
--
4 45
--
134
Northern Ireland
15
--
--
--
--
15
Poland
16 11
--
--
--
27
Spain
47 2
--
--
--
49
United Kingdom
58
--
--
--
--
58
Uruguay
156 35 179 208
--
578
Total 1,780 452 183 253 8 2,676
Data Source: FSIS Data Warehouse and PHIS databases.
33
Table 13. FY 2016 Number of Import Residue Samples Analyzed, by Exporting Country and Production Type
Country
Production Type
Beef Pork Veal Lamb Mutton Goat Chicken Turkey
Total
Fresh Processed Fresh Processed Fresh Fresh Fresh Fresh Processed Fresh Processed
Australia
77 8
-- --
20 26
37
-- -- -- --
168
Brazil
--
63 64
-- -- -- -- -- -- -- --
127
Canada
131 32 92 72 75 11
--
166 14 42 29
664
Chile
6
--
23
-- -- -- --
38 10 75
--
152
Costa Rica
12
-- -- -- -- -- -- -- -- -- --
12
Denmark
-- --
24 9
-- -- -- -- -- -- --
33
Finland
-- --
3
-- -- -- -- -- -- -- --
3
France
-- --
v 2
-- -- -- -- -- -- --
2
Germany
-- -- --
12
-- -- -- -- -- -- --
12
Iceland
-- -- -- -- --
48
-- -- -- -- --
48
Ireland
89
--
14
-- -- -- -- -- -- -- --
103
Israel
-- -- -- -- -- -- -- --
14
--
71
85
Italy
-- -- --
11
-- -- -- -- -- -- --
11
Japan
37
-- -- -- -- -- -- -- -- -- --
37
Korea, Republic
Of
-- -- -- -- -- -- -- --
1
-- --
1
Lithuania
5 12
--
18
-- -- -- -- -- -- --
35

34
Table 13. FY 2016 Number of Import Residue Samples Analyzed, by Exporting Country and Production Type (Cont.)
Country
Production Type
Beef Pork Veal Lamb Mutton Goat Chicken Turkey
Total
Fresh Processed Fresh Processed Fresh Fresh Fresh Fresh Processed Fresh Processed
Mexico
152 6 16 3
-- --
5
--
4
--
9
195
Netherlands
-- --
12
--
4
-- -- -- -- -- --
16
New Zealand
24 12
-- --
38 19 18
-- -- -- --
111
Nicaragua
134
-- -- -- -- -- -- -- -- -- --
134
Northern Ireland
-- --
15
-- -- -- -- -- -- -- --
15
Poland
-- --
16 11
-- -- -- -- -- -- --
27
Spain
-- --
47 2
-- -- -- -- -- -- --
49
United Kingdom
-- --
58
-- -- -- -- -- -- -- --
58
Uruguay
542 35
-- --
1
-- -- -- -- -- --
578
Total 1,209 168 384 140 138 104 60 204 43 117 109 2,676
Data Source: FSIS Data Warehouse and PHIS databases.

35
Table 14. FY 2016 Number of Chemical Analyates Tested Per Exporting Country and Production Type
Country
Production Type
Beef Pork Veal Lamb Mutton Goat Chicken Turkey
Total
Fresh Processed Fresh Processed Fresh Fresh Fresh Fresh Processed Fresh Processed
Australia
4,449 60
-- --
1,412 2,235 3,249
-- -- -- --
11,405
Brazil
--
366 5,123
-- -- -- -- -- -- -- --
5,489
Canada
8,662 174 7,695 414 5,973 844
--
11,974 63 3,337 161
39,297
Chile
399
--
2,124
-- -- -- --
2,955 11 6,185
--
11,674
Costa Rica
650
-- -- -- -- -- -- -- -- -- --
650
Denmark
-- --
1,910 56
-- -- -- -- -- -- --
1,966
Finland
-- --
315
-- -- -- -- -- -- -- --
315
France
-- -- --
12
-- -- -- -- -- -- --
12
Germany
-- -- --
78
-- -- -- -- -- -- --
78
Iceland
-- -- -- -- --
3,894
-- -- -- -- --
3,894
Ireland
5,218
--
1,161
-- -- -- -- -- -- -- --
6,379
Israel
-- -- -- -- -- -- -- --
78
--
132
210
Italy
-- -- --
63
-- -- -- -- -- -- --
63
Japan
2,224
-- --
-- -- -- -- -- -- --
2,224
Korea, Republic
Of
-- -- -- -- -- -- -- --
1
-- --
1
Lithuania
320 96
--
145
-- -- -- -- -- -- --
561

36
Table 14. FY 2016 Number of Chemical Analyates Tested Per Exporting Countries and Production Type (Cont.)
Country
Production Class
Beef Pork Veal Lamb Mutton Goat Chicken Turkey
Total
Fresh Processed Fresh Processed Fresh Fresh Fresh Fresh Processed Fresh Processed
Mexico
9,262 30 1,314 23
-- --
384
--
20
--
25
11,058
Netherlands
-- --
1,129
--
293
-- -- -- -- -- --
1,422
New Zealand
1,521 62
-- --
2,658 1,740 1,652
-- -- -- --
7,633
Nicaragua
9,369
-- -- -- -- -- -- -- -- -- --
9,369
Northern Ireland
-- --
1,438
-- -- -- -- -- -- -- --
1,438
Poland
-- --
1,331 61
-- -- -- -- -- -- --
1,392
Spain
-- --
3,820 12
-- -- -- -- -- -- --
3,832
United Kingdom
-- --
4,956
-- -- -- -- -- -- -- --
4,956
Uruguay
43,907 177
-- --
88
-- -- -- -- -- --
44,172
Total 85,981 965 32,316 864 10,424 8,713 5,285 14,929 173 9,522 318 169,490
Note: Multiple violative analytes in different tissue types may be associated with a single sample (Carcass).
Data Source: FSIS Data Warehouse and PHIS databases.

37
Table 15. FY 2016 Number of Samples and Chemical Residues under the Import
Residue Sample Program, by Exporting Country
Country Number of Samples
Samples with
Detected
Non-Violative
Samples with
Residue Detected
Violative
Chemical Residues
Analysis*
Australia 168
--
--
11,405
Brazil 127 8
--
5,489
Canada 664 1
--
39,297
Chile 152
--
--
11,674
Costa Rica 12
--
--
650
Denmark 33
--
--
1,966
Finland 3
--
--
315
France 2
--
--
12
Germany 12
--
--
78
Iceland 48
--
--
3,894
Ireland 103
--
--
6,379
Israel 85
--
--
210
Italy 11
--
--
63
Japan 37
--
--
2,224
Korea, Republic Of 1
--
--
1
Lithuania 35
--
--
561
Mexico 195 2
--
11,058
Netherlands 16
--
--
1,422
New Zealand 111
--
--
7,633
Nicaragua 134
--
2
9,368
Northern Ireland 15
--
--
1,438
Poland 27
--
--
1,392
Spain 49
--
--
3,832
United Kingdom 58
--
--
4,956
Uruguay 578 1 20
44,172
TOTAL 2,676 12 22
169,490
Note: * Multiple violative analytes in different tissue types may be associated with a single
sample (Carcass).
Data Source: FSIS Data Warehouse and PHIS databases.

38
Table 16. FY 2016 Import Residue Sample Program (Non-Violative and Violative)
Results, by Exporting Countries, Chemical Residues and Production Class
Country Chemical Residue
Veal
Beef
Residue Detected
Non-Violative
Residue Detected
Non-Violative
Residue
Detected
Violative
Brazil
Doramectin
--
1
--
Ivermectin
--
7
--
Canada
Sulfamethazine
1
--
--
Mexico
Ivermectin
--
1
--
Levamisole
--
1
--
Nicaragua
Ethion
--
--
2
Uruguay
Diazinon
--
--
1
Ethion
--
--
19
Ivermectin
--
1
--
Total 1 11 22
Note: Multiple violative analytes in different tissue types may be associated with a single sample
(Carcass).Data Source: FSIS Data Warehouse and PHIS databases.

39
Appendix I
NRP Non-Violative Positive and Violative Residue Samples Results
In addition to the publication of the FY2016 United States National Residue Program samples results,
FSIS will post the detailed positive non-violative, and positive violative residue results associated with
the NRP sampling program in a spreadsheet format on the FSIS website:
https://www.fsis.usda.gov/wps/portal/fsis/topics/data-collection-and-reports/chemistry/red-
books/red-book
This sheet includes detailed information regarding samples taken by FSIS in both the “scheduled”
sampling and the “inspector-generated” sampling. FSIS plans to publish this detailed results on an
ongoing basis. The purpose is to provide the residue testing results, and to increase program transparency
for all stakeholders. The detailed results include :sample collection and reviewed date, the project code,
the animal class, tissue type, chemical residue name, concentration value, sample results (whether
positive non-violative or postive violative), chemcial concentration values (if any) and the CFR reference
per chemical listed in the data sheet.
Appendix II
Statistical Table
Scheduled sampling is done to provide some assurance of detection of a violation that affects a given
percentage of the sample population.
Prior to FY 2012, FSIS tested 230 to 300 samples from each production class/residue compound class
pairing to obtain results that were statistically meaningful. The testing sample sizes of 230 or 300 ensured
FSIS a 90 percent or 95 percent probability, respectively, of detecting at least one chemical residue
violation if the violation rate is equal to or greater than one percent in the population being sampled.
Starting in FY 2012, FSIS stated in its residue sampling plan that the sample size selected/tested would
increase to about 800 samples for each of the nine major production class tested under Tier 1.
The statistical table provides the calculated number of samples required to ensure detection of at least one
violation that affects a given percentage of the sampled population. Statistically, for a binomial
distribution with sample size “n” and violation rate “v” (in decimal), if v is the true violation rate in the
population and n is the number of samples, the probability, p, of finding at least one violation among the
n samples (assuming random sampling) is p =
1 − (1 − v)
n
For example, if the true violation rate is 1% the probability of detecting at least one violation with sample
sizes of 230,300,390,460, and 800 are 90%, 95%, 98%, 99%,and 99.97% respectively.

40
In the table below the probability of detecting at least one violation with a sample size of 800 is italicized
and bolded.
Statistical Table – 2016 U.S. National Residue Program
Percentage %
Violative in the
population (v)
Number of samples required to detect
at least one violation in (n) samples
with a probability (p)
0.90
0.95
0.98
0.99
0.9997
Sample Size required “n”
10
22
29
37
44
77
5
45
59
76
90
158
1
230
300
389
459
807
0.57
403
525
684
806
1,419
0.50
460
598
780
919
1,618
0.37
620
808
1,055
1,242
2,188
0.29
793
1,032
1,347
1,586
2,793
0.10
2,302
2,995
3,910
4,603
8,108
The procedure to calculate the required sample size needed:
n
vp
)1
(1
−−=
Probability of detecting at least one violation in n sample of binomial
distribution with violation rate v
n
vp )1(1 −=−
Subtract one from both side of the equation. This gives the probability
of detecting No violations in n samples
n
vp )1log()1
log( −=−
Apply logarithmic function to both side of the equation
)
1log(*)1log( v
np −=−
A logarithmic function property
)1log(
)1log(
v
p
n
−
−
=
Sample size based on violation rate (v) and probability of detecting
(p)

41
Appendix III
List of Chemical Residues by Class/Method
i. Veterinary Drugs
For 2016 domestic sampling, FSIS has scheduled the following classes of veterinary drug analytes:
Multi-residue method
2-Aminosulfone
Albendazole
DCCD Gamithromycin Oxytetracycline Sulfamethoxypyridazine
2-Amino-
Flubendazole
Desethylene
Ciprofloxacin
Haloperidol Penicillin G Sulfanitran
2-Quinoxaline
Carboxylic Acid
(QCA)
Diclofenac Ipronidazole Phenylbutazone Sulfapyridine
Abamectin
Dicloxacillin
Ipronidazole - OH
Pirlimycin
Sulfaquinoxaline
Acepromazine
Difloxacin
Ketamine
Prednisone
Sulfathiazole
Albendazole
Dimetridazole
Ketoprofen
Ractopamine
Tetracycline
Amoxicillin
Dimetridazole -
OH
Levamisole Ronidazole Thiabendazole
Ampicillin
Dipyrone
Lincomycin
Salbutamol
Tildipirosin
Azaperone Doramectin
Melengestrol
Acetate
Sarafloxacin Tilmicosin
Butorphanol
Doxycycline
Meloxicam
Selamectin
Tolfenamic Acid
Carazolol
Emamectin
Benzoate
Metronidazole Sulfachloropyridazine Tulathromycin A
Cefazolin Enrofloxacin
– Metronidazole-
OH
Sulfadiazine Tylosin
Chloramphenicol
Eprinomectin
Morantel tartrate
Sulfadimethoxine
Tyvalosin
Chlortetracycline
Erythromycin A
Moxidectin
Sulfadoxine
Virginiamycin
Cimaterol
Fenbendazole
Nafcillin
Sulfaethoxypyridazine
Xylazine
Ciprofloxacin
Fenbendazole
sulphone
Norfloxacin Sulfamerazine Zeranol (β-Zearalanol)
Clindamycin
Florfenicol
Orbifloxacin
Sulfamethazine
Cloxacillin
Flubendazole
Oxacillin
Sulfamethizole
Danofloxacin
Flunixin
Oxyphenylbutazone
Sulfamethoxazole
Aminoglycoside Method
Amikacin
Gentamicin
Neomycin
Apramycin
Hygromycin B
Spectinomycin
Dihydrostreptomycin
Kanamycin
Streptomycin
Hormones Method
Megestrol
Melengestrol
Acetate
Hexestrol Zeranol

42
Beta-Agonist Method
Cimaterol
Ractopamine
Zilpaterol
Clenbuterol
Salbutamol
Avermectin Method
Doramectin
Ivermectin
Moxidectin
Nitrofuran Method
3-Amino-2-oxazolidinone
(AOZ)
1-Aminohydantoin (AHD) Semicarbazide (SEM)
3-Amino-5-morpholinomethyl-2-
oxazolidinone (AMOZ)
Carbadox Method
Quinoxaline-2-carboxylic acid

43
ii. Pesticides and environmental contaminants
a. Pesticide Method
1-Naphthol Coumaphos O
Fluroxypyr-1-
Methylhepyl-Ester
Pentachlorobenzen
e (PCB)
3-Hydroxycarbofuran Coumaphos S Fluvalinate
Permethrin
(cis&trans)
Acephate
DDD o,p’
Heptachlor
Piperonyl butoxide
Acetamiprid
DDD p,p’ + DDT,
o,p'
Heptachlor epoxide
(cis+ trans) or (B+A)
Pirimiphos methyl
Alachlor DDE o,p’
Hexachlorobenzene
(HCB)
Prallethrin
Aldicarb
DDE p,p’
Hexazinone
Profenofos
Aldicarb sulfone
DDT p,p’
Hexythiazox
Pronamide
Aldicarb sulfoxide
Deethylatrazine
Imazalil
Propachlor
Aldrin
Diazinon
Imidacloprid
Propanil
Atrazine
Dichlorvos (DDVP)
Indoxacarb
Propetamphos
Azinphos methyl
Dieldrin
Lindane (BHC gamma)
Propiconazole
Azoxystrobin
Difenoconazole
Linuron
Pyraclostrobin
Benoxacor
Diflubenzuron
Malathion
Pyrethrin I
Bifenthrin
Dimethoate
Metalaxyl
Pyrethrin II
Boscalid
Diuron
Methamidophos
Pyridaben
Buprofezin
Endosulfan I
Methomyl
Pyriproxyfen
Carbaryl Endosulfan II Methoxyfenozide
Resmethrin
(cis&trans)
Carbofuran
Endosulfan sulfate
Metolachlor
Simazine
Carfentrazone ethyl
Ethion
Metribuzin
Sulprofos
Chlordane cis Ethion monoxon
MGK-264 (isomers 1 &
2)
Tebufenozide

44
Chlordane trans
Ethofumesate
Myclobutanil
Tefluthrin
Chloroneb
Fenoxaprop ethyl
Nonachlor cis
Tetrachlorvinphos
Chlorothalonil
Fenpropathrin
Nonachlor trans
Tetraconazole
Chlorpropham
Fipronil
Norflurazon
Thiabendazole
Chlorpyrifos
Fipronil desulfinyl
Omethoate
Thiamethoxam
Chlorpyrifos methyl
Fipronil sulfide
Oxychlordane
Thiobencarb
Clothianidin Fluridone
Pentachloroaniline
(PCA)
Trifloxystrobin
1-Naphthol Coumaphos O
Fluroxypyr-1-
Methylhepyl-Ester
Pentachlorobenzen
e (PCB)
3-Hydroxycarbofuran Coumaphos S Fluvalinate
Permethrin
(cis&trans)
Acephate DDD o,p’ Heptachlor Piperonyl butoxide
Acetamiprid
DDD p,p’ + DDT,
o,p'
Heptachlor epoxide (cis+
trans) or (B+A)
Pirimiphos methyl
Alachlor DDE o,p’
Hexachlorobenzene
(HCB)
Prallethrin
Aldicarb
DDE p,p’
Hexazinone
Profenofos
Aldicarb sulfone
DDT p,p’
Hexythiazox
Pronamide
Aldicarb sulfoxide
Deethylatrazine
Imazalil
Propachlor
Aldrin
Diazinon
Imidacloprid
Propanil
Atrazine
Dichlorvos (DDVP)
Indoxacarb
Propetamphos
Azinphos methyl
Dieldrin
Lindane (BHC gamma)
Propiconazole
Azoxystrobin Difenoconazole Linuron Pyraclostrobin
Benoxacor
Diflubenzuron
Malathion
Pyrethrin I
Bifenthrin
Dimethoate
Metalaxyl
Pyrethrin II
Boscalid Diuron Methamidophos Pyridaben
Buprofezin Endosulfan I Methomyl Pyriproxyfen
Carbaryl Endosulfan II Methoxyfenozide
Resmethrin
(cis&trans)
Carbofuran
Endosulfan sulfate
Metolachlor
Simazine
Carfentrazone ethyl
Ethion
Metribuzin
Sulprofos
Chlordane cis Ethion monoxon
MGK-264 (isomers 1 &
2)
Tebufenozide
Chlordane trans
Ethofumesate
Myclobutanil
Tefluthrin
Chloroneb
Fenoxaprop ethyl
Nonachlor cis
Tetrachlorvinphos
Chlorothalonil
Fenpropathrin
Nonachlor trans
Tetraconazole
Chlorpropham
Fipronil
Norflurazon
Thiabendazole
Chlorpyrifos
Fipronil desulfinyl
Omethoate
Thiamethoxam

45
Chlorpyrifos methyl Fipronil sulfide Oxychlordane Thiobencarb
Clothianidin Fluridone
Pentachloroaniline
(PCA)
Trifloxystrobin
b. Metals Method
Aluminum (Al)
Copper (Cu)
Selenium (Se)
Barium (Ba)
Iron (Fe)
Strontium (Sr)
Boron (B)
Lead (Pb)
Thallium (Tl)
Cadmium (Cd)
Manganese (Mn)
Vanadium (V)
Chromium (Cr)
Molybdenum (Mo)
Zinc (Zn)
Cobalt (Co)
Nickel (Ni)

46
Appendix IV
U.S. NRP – Domestic Scheduled Sampling Program
Year Number of Samples
Number of Violative
Samples
Number of Non-
Violative Positive
Analytes
Number of
Violative Chemical
Residues
* FY2013
4,583
19
23
8
FY2014 6,066 10 34 10
FY2015 6,445 12 23 8
FY2016 7,067 26 24 11
* Note: FSIS moved to a fiscal evaluation period beginning with FY12. FY 2013 covers only Jan-Sept, 2013.
Appendix V
U.S. NRP – Import Re-inspection Sampling Program
Year
Number of
Samples
Number of Violative Samples
Violative
Residues
* FY2013
817
4
Avermectins
FY2014 1,967 8
Ivermectin (7),
Zilpaterol (1)
FY2015 2,922 7
Abamectin (1) Ethion (5),
Piperonyl Butoxide (1)
FY2016 2,676 22
Ethion (21),
Diazinon (1)
* Note: FSIS moved to a fiscal evaluation period beginning with FY12. FY 2013 covers only Jan-Sept, 2013.

47
Appendix VI
NRP – Domestic Inspector Generated Sampling Program (include KIS™ test) & lab confirmed residue results
Year
Number of
Samples
/
(Include In-plant
KIS™ Screens
Tests)
Number of Samples
Tested in FSIS Labs
/
(include in-plant KIS™
screens positive)
Number of Lab-
Confirmed
Violative
Analytes
/ Number of
Violative
Carcasses
Top Three
Violative
Chemical
Residue
Number of
Lab-
Confirmed
Non-
Violative
Positive
Analytes
Top Three Non-
Violative Chemical
Residue
*FY2013
170,692 /
(170,560)
4,100 /
(3,968)
1,265 /
1,053
Ceftiofur
Penicillin
Neomycin
1,099
Oxytetracyline
Neomycin
Ceftiofur
FY2014
210,705 /
(210,516)
5,048 /
(4,859)
1,408 /
1,136
Ceftiofur
Penicillin
Neomycin
1,150
Oxytetracyline
Tulathromycin
Penicillin
FY2015
184,167 /
(184,010)
4,179 /
(4,022)
1,024 /
796
Ceftiofur
Penicillin
Sulfamethazine
873
Tulathromycin
Oxytetracyline
Neomycin
FY2016
182,313 /
(182,184)
3,778 /
(3,649)
893 /
732
Ceftiofur
Penicillin
Sulfadimethoxine
728
Oxytetracycline
Tulathromycin
Penicillin
Note:
• (Number of KIS™ test samples in paranthesis)
• Multiple violative analytes in different tissue types may be associated with a single sample (Carcass).
• FSIS moved to a fiscal evaluation period beginning w/FY13. FY 2013 covers Jan-Sept, 2013 only.

48
Appendix VII
2016 FSIS Residue Sampling for Siluriformes
On December 2, 2015, FSIS published the final rule, “Mandatory Inspection of Fish of the
Order
Siluriformes and Products Derived From Such Fish.” The 2008 Farm Bill amended the Federal Meat
Inspection Act (FMIA), to make Siluriformes a species amendable to the FMIA and therefore, subject to
FSIS inspection. FSIS is providing an 18 month transitional period for the inspection of Siluriformes and
the residue testing will be done based on parameters set forth in
the final rule. During the first 18 months,
FSIS will schedule routine testing of Siluriformes for dyes (malachite
green and gentian violet),
nitrofurans, veterinary drugs, metals, and
pesticides residues.
Note: The sampling scheme may change during the 18 month transitional period based on sampling
results and findings by FSIS.
Domestic
Imports
Total
Siluriformes
77
84
161
Siluriformes
Chemical Class
May 2015- Sep 2016
Dyes Metals MRM Nitrofurans Pesticides
Domestic
√
√
√
√
√
Imports
√
√
√
√
√
Table 17. FY2016 NRP Residue Scheduled Samples -Number of Residue Samples
Tested Per Chemical Method by Sampling Plan
Siluriformes
(# Samples
Collected)
Number of Samples per Chemical Method
Dyes Metals MRM Nitrofurans Pesticides
Domestic (77)
31 (1)
31
77
46
46
Import (84)
42
42
42 (1)
42 (1)
42
Total (161)
73
73
119
88
88
Note: Number of violative samples (in parenthesis)
Data Source: FSIS Data Warehouse and PHIS databases.

49
Table 18. FY 2016 NRP Residue Scheduled Samples - Number of Chemical Analytes
Tested Per Chemical Method by Sampling Plan
Siluriformes
(# Samples
Collected)
Number of Chemical Analytes per Chemical Method
Dyes Metals MRM
Nitrofuran
s
Pesticides Total
Domestic (77)
154
821
14,283
230
7,338
22,826
Import
(84)
203 1181 8,105 210 6,274 15,973
Total
(161)
357 2,002 22,388 440 13,612 38,799
Note: Multiple analytes may be associated with the same sample. Not all samples are tested for all
chemical method. Number of samples per chemical method is indicated in Table 4
Data Source: FSIS Data Warehouse and PHIS databases.
Table 19. FY 2016 NRP Siluriformes Residue Inspection Program Violations
Animal Sampling Compound Concentration Units
Tolerance
Level
Value
Authority
(CFR
Citation)
Siluriformes
Domestic Crystal Violet 0.162 ppm
Siluriformes
Import Enrofloxacin 22.500 ppm
Siluriformes
Import Gentian Violet 16.1 ppb

