
Evidence-Based Clinical Practice Guideline
Comprehensive
Pediatric Eye and
Vision Examination

2
The American Optometric Association represents approximately 39,000 doctors of optometry, optometry students
and paraoptometric assistants and technicians. Optometrists serve individuals in nearly 6,500 communities across
the country, and in 3,500 of those communities are the only eye doctors. Doctors of optometry provide two-thirds of
all primary eye care in the United States.
Doctors of optometry are on the frontline of eye and vision care. They examine, diagnose, treat, and manage
diseases and disorders of the eye. In addition to providing eye and vision care, optometrists play a major
role in an individual’s overall health and well-being by detecting systemic diseases such as diabetes and
hypertension.
The mission of the profession of optometry is to fulfill the vision and eye care needs of the public through
clinical care, research, and education, all of which enhance the quality of life.
Disclosure Statement
This Clinical Practice Guideline was funded by the American Optometric Association (AOA) without financial support
from any commercial sources. The Evidence-Based Optometry Guideline Development Group and other guideline
participants provided full written disclosure of conflicts of interest prior to each meeting and prior to voting on the
quality of evidence or strength of clinical recommendations contained within this guideline.
Disclaimer
Recommendations made in this guideline do not represent a standard of care. Instead, the recommendations are
intended to assist the clinician in the decision-making process. Patient care and treatment should always be based
on a clinician’s independent professional judgment, given the patient’s circumstances, and in compliance with state
laws and regulations.
The information in this guideline is current to the extent possible at the time of publication.
OPTOMETRY: THE PRIMARY EYE CARE PROFESSION

3
EVIDENCE-BASED CLINICAL PRACTICE GUIDELINE
COMPREHENSIVE PEDIATRIC EYE
AND VISION EXAMINATION
Developed by the AOA Evidence-Based Optometry Guideline Development Group
Approved by the AOA Board of Trustees February 12, 2017
©American Optometric Association 1995, 2002, 2015
243 N. Lindbergh Blvd., St. Louis, MO 63141-7881

4
TABLE OF CONTENTS
EVIDENCE-BASED CLINICAL GUIDELINE
A. What is the Evidence-Based Process? .................5
B. How to Use This Guideline ....................................7
I. INTRODUCTION .......................................................10
A. Guideline Objectives ............................................10
II. BACKGROUND .........................................................10
A. Visual Development ..............................................10
B. Epidemiology of Eye and Vision Disorders in
Children .................................................................11
C. Access to Care .....................................................15
D. Costs of Eye and Vision Disorders in Children .. 16
E. Early Detection and Prevention of Eye and Vision
Disorders ..............................................................16
III. CARE PROCESS ..................................................... 17
A. Comprehensive Pediatric Eye and Vision
Examination ..........................................................17
1. General Considerations ....................................... 17
a. Infants and Toddlers .....................................18
b. Preschool Children ....................................... 18
c. School-age Children ..................................... 18
2. Examination Procedures .....................................18
3. Patient History.....................................................19
4. Testing ................................................................19
4.1 Testing of Infants and Toddlers .......................... 19
a. Visual Acuity ................................................. 19
b. Refraction ....................................................19
c. Binocular Vision and Ocular Motility .............. 20
4.2 Testing of Preschool Children ............................21
a. Visual Acuity ................................................. 21
b. Refraction ....................................................21
c. Binocular Vision, Ocular Motility, and
Accommodation..........................................22
d. Color Vision ..................................................22
4.3 Testing of School-age Children ..........................22
a. Visual Acuity ................................................. 22
b. Refraction ....................................................23
c. Binocular Vision, Ocular Motility, and
Accommodation..........................................23
d. Color Vision ..................................................24
5. Ocular and Systemic Health Assessment ...........24
a. Assessment of Pupillary Responses .............25
b. Visual Field Evaluation ..................................25
c. Evaluation of the Ocular Anterior Segment and
Adnexa .......................................................25
d. Evaluation of the Ocular Posterior Segment ..25
e. Measurement of Intraocular Pressure ...........25
6. Supplemental Testing .......................................... 25
a. Electrodiagnostic Testing .............................. 25
b. Imaging ........................................................ 25
c. Testing for Learning-related Vision Problems 26
7. Children with Special Needs ................................26
a. At-risk Children .............................................26
b. Developmental Disabilities ............................27
8. Trauma and Ocular Manifestations of Child Abuse/
Neglect ............................................................27
a. Trauma (Accidental) ...................................... 27
b. Ocular Manifestations of Child Abuse and
Neglect (Non-accidental) ............................. 27
9. Potential Benefits and Harms of Testing ..............29
B. Assessment and Diagnosis .................................29
C. Management ........................................................ 29
1. Prescription for Correction...................................29
2. Additional Treatment Services .............................29
3. Counseling and Education ..................................29
a. Eye Safety and Protection ............................31
b. Ultraviolet Radiation and Blue Light
Protection ...................................................32
c. Impact of Near Work and Reduced Time
Outdoors on Vision .....................................32
d. Myopia Control .............................................33
4. Coordination and Frequency of Care ................... 33
a. Coordination of Care ....................................33
b. Frequency of Care ........................................34
c. At-risk Children .............................................39
D. Conclusion ............................................................39
IV. REFERENCES ..........................................................41
V. APPENDIX .................................................................55
A. Appendix Figure 1: Comprehensive Pediatric Eye
and Vision Examination: A Flowchart ................55
B. Appendix Table 1: Potential Components of the
Comprehensive Eye and Vision Examination for
Infants and Toddlers ............................................56
C. Appendix Table 2: Potential Components of the
Comprehensive Eye and Vision Examination for
Preschool Children ..............................................57
D. Appendix Table 3: Potential Components of the
Comprehensive Eye and Vision Examination for
School-age Children ............................................58
E. Appendix Table 4: Partial Listing of Ocular
Manifestations of Neurodevelopmental Disorders
and Other Syndromes ..........................................59
F. Abbreviations/Acronyms ......................................61
G. Summary of Action Statements .......................... 62
H. Gaps in Research Evidence .................................64
VI. METHODOLOGY FOR GUIDELINE
DEVELOPMENT .......................................................64
VII. EVIDENCE-BASED OPTOMETRY GUIDELINE
DEVELOPMENT GROUP ......................................... 66

5
EVIDENCE-BASED CLINICAL GUIDELINES
A. WHAT IS THE EVIDENCE-BASED PROCESS?
As a result of the Medicare Improvement for Patients and Providers Act of 2008, Congress commissioned the
Secretary of Health and Human Services to create a public-private program to develop and promote a common
set of standards for the development of clinical practice guidelines (CPGs). These standards address the structure,
process, reporting, and final products of systematic reviews of comparative effectiveness research and evidence-
based clinical practice guidelines.
The Institute of Medicine (IOM), now the Health and Medicine Division of the National Academies of Sciences,
Engineering, and Medicine (NASEM), in response to a request from the Agency for Healthcare Research and Quality
(AHRQ), issued two reports in March 2011: Clinical Practice Guidelines We Can Trust and Finding What Works in
Health Care: Standards for Systematic Reviews.
In Clinical Practice Guidelines We Can Trust,
1
the IOM redefined CPGs as follows
“Clinical practice guidelines are statements that include recommendations intended to optimize patient care that
are informed by a systematic review of the evidence and an assessment of the benefits and harms of alternative
care options.”
The report states that to be trustworthy, guidelines should:
• Be based on a systematic review of existing evidence
• Be developed by a knowledgeable, multidisciplinary panel of experts and key stakeholders
• Consider important patient subgroups and preferences, as appropriate
• Be based on a transparent process that minimizes conflicts of interest and biases
• Provide a clear explanation of the logical relationships between alternative care options and health outcomes
• Provide a grading of both the quality of evidence and the strength of the clinical recommendation
• Be revised as appropriate when new evidence warrants modifications of recommendations.
Based on the IOM/NASEM reports, the American Optometric Association (AOA) Evidence-Based Optometry (EBO)
Committee developed a 14-step process to meet the new evidence-based recommendations for trustworthy
guidelines.

6
AOA’s 14 Steps to Evidence-Based Clinical Practice Guideline Development
1. Guideline Development Group: Evidence-Based Optometry (EBO) Committee selects a multidisciplinary panel of
experts, including patient and public representatives, for the Guideline Development Group (GDG).
2. Transparency and COI: GDG manages all conflict of interest (COI), which is documented by AOA staff.
3. Clinical Questions*: GDG explores and defines all clinical questions through a Question Formulation Meeting and
defines search criteria.
4. Search for Evidence: AOA Staff sends clinical questions for query (outside researchers) and provides all papers to
the Guideline Development Reading Group (GDRG). There should be no inclusion of Systematic Review (SR) writers in
the GDRG.
5. Grade Evidence and Clinical Recommendations: Two clinicians from the GDRG read and grade papers,
randomly selected according to the pre-designed evidence search criteria. They state clinical recommendation(s) from
each paper and grade the strength of each.
6. Articulate Clinical Recommendations*: GDRG reviews all clinical recommendations and articulates each for
inclusion in the guideline during an “Articulation of Recommendations” meeting and identified gaps in medical research
are documented.
7. Write Draft: AOA Staff sends the Articulation results to the writer for development of draft 1.
8. Draft Review and Edits*: GDG reads draft 1, discusses and edits.
9. Rewrite/Final Drafts: AOA Staff sends the draft results to the writer for writing/revisions for draft 2, then sends to
medical editor for copy editing, then a final review is completed as necessary.
10. Approval for Peer Review: AOA Staff or EBO Committee Chair sends the Peer Review draft to AOA Board of
Trustees for approval to post for peer and public review. This draft is posted on the AOA website, the review period is
announced, and comments are solicited.
11. Final Document Produced: GDG reviews all peer review comments and revises the final document (includes peer
review comments, documents why a peer review comment was not included, or identifies further gaps for review when
preparing the next edition).
12. Final Draft Approval and Legal Review: AOA Staff or EBO Committee Chair sends to the AOA Board of
Trustees and AOA Legal Counsel for approval that the GDG followed the evidence-based process as outlined by the
IOM and AOA EBO Committee (same management of COI).
13. Post Guidelines: AOA Staff posts the evidence-based guideline to AOA website and submits it to the National
Guideline Clearinghouse for public use, accompanied by AOA’s written process and documents.
14. Schedule Reviews: GDG reviews all previously identified gaps in medical research and any new evidence, and
revises the evidence-based guideline every 2 to 5 years.
**Denotes face-to-face meeting

7
B. HOW TO USE THIS GUIDELINE
The following table provides the grading system used in this guideline for rating evidence-based clinical statements.
Grades are provided for both quality of the evidence and strength of clinical recommendations.
Key to Quality of Evidence and Strength of Clinical Recommendation Grading
Grade Quality of Evidence Levels
A
Data derived from well-designed, randomized clinical trials (RCTs); systematic reviews; meta-analyses; or
diagnostic studies (Grade A) of relevant populations with a validated reference standard. Grade A diagnostic
studies do not have a narrow population or use a poor reference standard and are not case control studies of
diseases or conditions.
B
Randomized clinical trials (RCTs) with weaker designs; cohort studies (retrospective or prospective); or
diagnostic studies (Grade B). Grade B diagnostic studies have only one of the following: a narrow population,
or the sample used does not reflect the population to whom the test would apply, or uses a poor reference
standard, or the comparison between the test and reference standard is not blinded, or are case control studies
of diseases or conditions.
C
Studies of strong design, but with substantial uncertainty about conclusions or serious doubts about
generalizations, bias, research design, or sample size. Nonrandomized trials; case control studies (retrospective
or prospective); or diagnostic studies (Grade C). Grade C diagnostic studies have at least 2 or more of the
following: a narrow population, or the sample used does not reflect the population to whom the test would
apply, or uses a poor reference standard, or the comparison between the test and reference standard is not
blinded, or are case control studies of diseases or conditions.
D
Cross sectional studies; case reports/series; reviews; position papers; expert opinion; or reasoning from
principal.
Strength of Clinical Recommendation Levels
Strong Recommendation: The benefits of the recommendation clearly exceed the harms (or the harms clearly exceed
the benefits in the case of a negative recommendation) and the quality of evidence is excellent (Grade A or B). In some
clearly identified circumstances, a strong recommendation may be made on lesser evidence when high-quality evidence
is impossible to obtain and the anticipated benefits strongly outweigh the harms.
This recommendation should be followed unless clear and compelling rationale for an alternative approach
is present.
Recommendation: The benefits of the recommendation exceed the harms (or the harms exceed the benefits in the
case of a negative recommendation) but the quality of evidence is not as strong (Grade B or C). In some clearly identified
circumstances, a recommendation may be made on lesser evidence when high-quality evidence is impossible to obtain
and the anticipated benefits strongly outweigh the harms.
This recommendation should generally be followed, but remain alert for new information.
Option: The benefits of the recommendation exceed the harms (or the harms exceed the benefits in the case of a
negative recommendation) but the quality of evidence is low (Grade D) or well-done studies (Grade A, B, or C) show little
clear advantage of one approach versus another. In some clearly identified circumstances, an option may be elevated
to a recommendation even with lesser evidence when high-quality evidence is impossible to obtain and the anticipated
benefits strongly outweigh the harms.
There should be an awareness of this recommendation, but a flexibility in clinical decision-making, as well as
remaining alert for new information.

8
Clinical Notes and Statements
Quality of evidence grades (A, B, C, or D) are shown throughout the guideline for clinical notes and statements. For
example, a clinical note or statement with a quality of evidence grade of “B” is shown as “(Evidence Grade: B)”.
Evidence-Based Action Statements will be highlighted in an “Action” box, with the quality of evidence, level of
confidence, and clinical recommendation grading information listed. For example:
EVIDENCE-BASED ACTION STATEMENT: Parents/caregivers and children should be educated about
potential risks for eye injuries at home, at school, and during sports and recreational activities and advised
about safety precautions to decrease the risk of ocular injury.
193,199
Prevention of eye injuries in children should
focus on the use of protective eyewear, parental supervision, and on education about both the risks of eye
injury and the benefits of protective eyewear.
194
Evidence Quality: Grade B: Retrospective cohort studies
Level of Confidence: Medium
Clinical Recommendation Strength: Strong Recommendation. This recommendation should be followed
unless clear and compelling rationale for an alternative approach is present.
Evidence Statements: It is important to discuss eye safety issues with children/ parents/caregivers.
193
(Evidence Grade: B),
199
(Evidence Grade: B)
Prevention strategies should focus on the use of protective eyewear, parental supervision, and on childhood
education about both the risks of eye injury and the utility of protective eyewear.
194
(Evidence Grade: B)
Potential Benefits: Reduction in eye injuries in
children
Potential Risks/Harms: None
Benefit and Harm Assessment: Benefits significantly outweigh harms
Potential Costs: Direct cost of counseling as part of a pediatric eye and vision examination
Value Judgments: None
Role of Patient Preferences: None
Intentional Vagueness: Specific type/form of counseling is not stated, as it is patient specific
Gaps in Evidence: None identified
The Action Statement profile provides additional information related to the development and implementation of the
clinical recommendation. The following is an explanation of the categories listed in the profile:
Evidence Quality – The quality of evidence grade (A, B, C, or D) or the aggregate quality of evidence grade (if
multiple studies were available for review) and the type/method of research study or studies reviewed.
Level of Confidence – The consistency of the evidence and the extent to which it can be trusted specified as
high, medium, or low.
Clinical Recommendation Strength – The grade (Strong Recommendation, Recommendation, or Option)
assigned to the implementation of the clinical recommendation made in the Action Statement.
Evidence Statements – The clinical statements derived from research studies reviewed that support the Action
Statement.

9
Potential Benefits – Favorable changes which would likely occur if the Action Statement was followed.
Potential Risks/Harms – Adverse effects or unfavorable outcomes that may occur if the Action Statement was
followed.
Benefit and Harm Assessment – A comparison of the relationship of benefits to harms specified as “benefits
significantly outweigh harms” (or vice versa) or a “balance of benefits and harms.”
Potential Costs – Direct and indirect costs refer to the costs of the procedure, test, or medication; time spent
counseling the patient; administrative time; parent/caregiver time off from work, etc.
Value Judgments – Determinations made by the Guideline Development Group in the development of the
Action Statement relating to guiding principles, ethical considerations, or other priorities.
Role of Patient Preference – The role the patient has in shared decision making regarding implementation of
the Action Statement specified as large, moderate, small, or none.
Intentional Vagueness – Specific aspects of the Action Statement that are left vague due to factors such as the
role of clinical judgment, patient variability, concerns over setting legal precedent, etc.
Gaps in Evidence – Areas identified during searches and evaluations of the research that show gaps in available
evidence.
Consensus-Based Action Statements, based on consensus by the Guideline Development Reading Group, are
also highlighted in an “Action” box, but without any quality of evidence or strength of clinical recommendation grading
information listed. For example:
CONSENSUS-BASED ACTION STATEMENT: At the conclusion of a comprehensive pediatric eye and vision
examination, the diagnosis should be explained to the patient/parent/caregiver and related to the patient’s
symptoms, and treatment plans and prognosis discussed.
Evidence Quality: There is a lack of published research to support or refute the use of this recommendation.
Benefit and Harm Assessment: Implementation of this recommendation is likely to increase patient/
parent/caregiver understanding of any diagnosed eye or vision problems and improve compliance with any
recommended treatment. The benefits of this recommendation were established by expert consensus opinion.

10
I. INTRODUCTION
Eye and vision problems in children are a significant
public health concern. An estimated one in five
preschool children have vision problems.
2-8
In the United
States, about one in four school-age children wear
corrective lenses.
9
Since eye and vision problems can
become worse over time, early diagnosis and treatment
are essential to optimize children’s eye health and vision
and to prevent future vision loss.
Eye and vision disorders can lead to problems in a child’s
normal development,
10,11
school performance,
12-16
social
interactions,
17
and self-esteem.
17-19
Vision disorders that
occur in childhood may manifest as problems well into
adulthood, affecting an individual’s level of education,
employment opportunities, and social interactions.
20
Early recognition of visual disorders is especially
important in children with developmental and intellectual
disabilities.
21,22
Children with disabilities are reported to
have significantly more eye and vision problems (e.g.,
strabismus, refractive errors, and nystagmus) than
children without these disabilities.
22-27
The increasing
severity of the disability may be related to a higher
prevalence of vision problems.
This Evidence-Based Clinical Practice Guideline for the
Comprehensive Pediatric Eye and Vision Examination
describes procedures for evaluation of the eye health
and vision status of infants and children. It contains
recommendations for timely diagnosis and, when
necessary, referral for consultation with, or treatment
by, another health care provider. Other guidelines
developed to address treatment of specific eye and
vision conditions can be found at AOA Clinical Practice
Guidelines web page.
The recommendations in this guideline were developed
to assist doctors of optometry and ophthalmologists
involved in providing eye and vision examinations for
infants and children. Others who assist in providing
coordinated patient care for specific services, as well as
patients, parents, and caregivers, may also gain insight
from this document.
A. GUIDELINE OBJECTIVES
This Guideline can help achieve the following objectives:
• Recommend an optimal timetable for comprehensive
eye and vision examinations for infants and children
(newborn through 18 years of age)
• Suggest appropriate procedures to effectively
examine the eye health, vision status, and ocular
manifestations of systemic disease of infants and
children
• Reduce the risks and adverse effects of eye and
vision problems in infants and children through
prevention, education, early diagnosis, treatment,
and management
• Inform and educate patients, parents/caregivers, and
other health care providers about the importance of
eye health and good vision, and the need for and
frequency of pediatric eye and vision examinations.
II. BACKGROUND
A. VISUAL DEVELOPMENT
Development of the visual system begins prenatally and
continues after birth.
28
Basic visual functions develop
rapidly during the first year of life. By 6 months of age,
vision has become the dominant sense and forms the
basis for perceptual, cognitive, and social development;
29
however, maturation of the visual system continues for
several years. From birth to about 6 years of age, the
visual system is susceptible to vision conditions that
cause either blurred visual input or abnormal binocular
interaction such as interference from amblyogenic
bilateral refractive error, amblyogenic anisometropia,
constant unilateral strabismus, congenital cataracts,
hemangioma, corneal scarring, and any other condition
that obstructs vision. This interference can lead to
amblyopia, which, if left untreated, can cause serious
vision loss.
Objective testing (visual evoked response) demonstrates
that the visual cortex is capable of achieving 20/20
visual acuity by 6 months of age;
30
however, the ability
of a child to respond to subjective visual acuity tests is
influenced by verbal and cognitive development. For

11
some children, it may not be possible to elicit 20/20
visual acuity until after 5 years of age; therefore, it is
critical to select age appropriate tests. Stereopsis first
appears at 3 to 4 months of age and continues to
develop through the first two years of life.
31, 32
Mature
accommodative behavior is present at 5 to 24 months of
age.
33
Development of accommodative facility, vergence
ability, and eye movements continues in the preschool
and school-age years.
34-37
B. EPIDEMIOLOGY OF EYE AND VISION
DISORDERS IN CHILDREN
There are many visual conditions and ocular or systemic
diseases, which may occur in childhood that can
affect visual development. Eye and vision disorders
experienced by infants and children may include:
• Refractive errors
Refractive errors (hyperopia, myopia, astigmatism,
and anisometropia) are the most common causes of
correctable reduced vision in children.
38, 39
Estimates of
refractive errors in children 6 months to 72 months (6
years) of age are shown in Table 1.
Hyperopia has a high prevalence among young children
up to 5 years old, with over 20% estimated to have
≥2.00 diopters (D).
2,3
Hyperopia (≥2.00D) is found to be a
significant risk factor for the development of strabismus
40
and amblyopia
41
up to 5 years of age.
Myopia generally develops in children during their early
school years and increases in magnitude, as they get
older. The age at onset typically ranges from 7 to 16
years. In the Collaborative Longitudinal Evaluation of
Ethnicity and Refractive Error Study (CLEERE), one in six
children ages 5 to 16 (Asian, Hispanic, African American,
Native American and White) developed myopia during
their school-age years. More than 75% of the new cases
of myopia occurred between the ages of 9 and 13.
42
Among school-age children, the prevalence of myopia
has been increasing in recent years and developing at
a younger age.
42,43
The National Health and Nutrition
Examination Survey results for 12 to 17 year olds show
the prevalence of myopia increased from 24% in 1971-
1972 to 33.9% in 1999-2004
44
and it continues to rise.
High levels of myopia can contribute to the development
of lattice degeneration, retinal holes, tears, or
detachment, cataracts, glaucoma, and myopic macular
degeneration.
45,46
Astigmatism up to 2.00D is common in children under
3 years of age. Studies show that 30 to 50% of infants
less than 12 months of age have astigmatism (≥1.00D),
which declines over the first few years of life, and
becomes stable by approximately 2 1/2 to 3 years of
age.
47, 48
Anisometropia of 1.00D or more is considered
clinically significant. There is a low prevalence (4%) of
anisometropia before 6 years of age;
49
however, it has
been shown to increase to nearly 6% at 12 to 15 years
of age. Infantile anisometropia can be transient and
may decrease; however, severe anisometropia (≥3.00D)
may persist and is likely to lead to the development of
amblyopia during the preschool years.
50, 51
Table 1: Prevalence of Refractive Errors in Children
6 Months to 72 Months (6 Years) of Age
Condition
White
Non-
Hispanic
Hispanic
African
American
Asian
Myopia
≤1.00D spherical
equivalent (SE)
1.2% 3.7% 6.6% 4.0%
≥1.00D SE 0.7% 5.5%
Hyperopia
≥2.00D SE 25.7% 26.9% 20.8% 13.5%
≥3.00D SE 8.9% 4.4%
Astigmatism
≥1.50D cylindrical
refractive error
6.3% 16.8% 12.7% 8.3%
≥3.00D cylindrical
refractive error
2.9% 1.0%
Anisometropia
≥1.00D SE 4.3% 4.2%

12
Source: Multi-Ethnic Pediatric Eye Disease
Study
2-4,49
and the Baltimore Pediatric Eye Disease
Study
5
(Note: The ethnicity of children reported in Tables 1, 2, 3
and 4 is based on the categorization used in the studies
cited.)
Table 2: Prevalence of Refractive Errors in Children
5 to 17 Years of Age
Condition
White
Non-
Hispanic
Hispanic
African
American
Asian
Myopia
≥0.75D in each
principal meridian
4.4% 13.2% 6.6% 18.5%
Hyperopia
≥1.25D in each
principal meridian
19.3% 12.7% 6.4% 6.3%
Astigmatism
≥1.00D difference
between two
principal meridians
26.4% 36.9% 20.8% 33.6%
Source: Collaborative Longitudinal Evaluation of
Ethnicity and Refractive Error Study
52
In the school-based CLEERE study of children 5 to 17
years of age, overall 9.2% of the children were myopic,
12.8% were hyperopic, and 28.4% had astigmatism
(Table 2).
Click to view the (AOA Clinical Practice Guidelines web
page)
• Amblyopia
Amblyopia is the leading cause of monocular vision loss
in children. It is generally attributable to strabismus,
anisometropia, combined strabismus and anisometropia,
or form deprivation (e.g., media opacity). Unilateral
amblyopia is commonly associated with constant
unilateral strabismus and/or amblyogenic anisometropia,
while bilateral amblyopia usually results from high bilateral
refractive error
53
or bilateral form deprivation.
Although amblyopia is a treatable condition in both
children and adults,
54
the end result is better when
diagnosed and treated early.
55-60
The prevalence of
amblyopia in the general population is believed to be
between 2% and 2.5%.
61
Estimates of the prevalence of
amblyopia in young children in an urban population are
shown in Table 3.
Click to view the (AOA Clinical Practice Guidelines web
page)
• Strabismus
The estimated prevalence of strabismus in the general
population varies from 2.5% to 4.6% based on various
studies.
62
The prevalence of strabismus in young children
in an urban population is shown in Table 3.
Although strabismus can develop at any age, it usually
develops during childhood. Infantile esotropia has
an onset prior to 6 months of age; accommodative
esotropia typically has an onset between 2 to 3 years
of age, but can develop before 6 months of age. Young
children with constant unilateral strabismus often develop
amblyopia and impaired stereopsis. Early identification
and treatment of children with strabismus may prevent
amblyopia and preserve stereopsis.
Table 3: Prevalence of Amblyopia and Strabismus
in Children 6 Months to 72 Months (6 Years) of Age
Condition
White Non-
Hispanic
Hispanic
African
American
Asian
Amblyopia 1.8% 2.6% 0.8% - 1.5% 1.8%
Strabismus 3.2% - 3.3% 2.4% 2.1% - 2.5% 3.6%
Source: Multi-Ethnic Pediatric Eye Disease Study
6, 8
and the Baltimore Pediatric Eye Disease Study
7
Click to view the (AOA Clinical Practice Guidelines web
page)
• Non-strabismic binocular vision problems and
accommodative disorders
Other than refractive errors, the most prevalent
vision conditions in children fall into the category of
accommodative and binocular vision anomalies, as

13
reported in a large-scale prospective study of the
prevalence of vision disorders and ocular disease in a
clinical population of children between the ages of 6
months and 18 years.
63
Oculomotor conditions
Oculomotor conditions include a variety of eye
movement disorders, which can affect saccadic,
fixation, and vergence eye movements.
Convergence insufficiency (CI) is a binocular vision
disorder that affects up to 8.3% of school-age
children
64
and is associated with symptoms such
as eyestrain, headaches, blurred vision, diplopia,
sleepiness, difficulty concentrating, movement
of print while reading, loss of place, and loss of
comprehension after short periods of reading.
65-67
The Convergence Insufficiency and Reading Study
Group investigators found that 13% of fifth and
sixth grade children (definite and high suspect)
had clinically significant CI (insufficient fusional
convergence, receded nearpoint of convergence,
and/or exophoria at near ≥4 prism diopters at far).
68
Convergence excess (CE) has been reported
to occur in 7.1% of children in one clinical
pediatric population.
63
It may be due to a high
accommodative convergence/accommodation
(AC/A) ratio. Symptoms can include blurred vision,
diplopia, headaches, and difficulty concentrating on
near tasks.
Accommodative disorders
Children with accommodative dysfunctions may
have difficulty focusing on near objects, maintaining
focus for long periods, or easily changing focus
from near to far and back again. Studies in clinic
populations have been conducted to determine
the prevalence of accommodative dysfunction.
A study of over 2,000 children found that 5% of
children between the ages of 6 and 18 years had
accommodative disorders.
63
Click to view the (AOA Clinical Practice Guidelines
web page)
• Color vision deficiency
Children with color vision deficiency, either inherited or
acquired, may have difficulty precisely matching colors
or discriminating fine color differences. Inherited (X
chromosome) color vision deficiency is estimated to
occur in nearly 8% of white males and less than 0.4% of
white females, with lower prevalence in other ethnicities
69
(Table 4). The severity of color vision deficiency can
range from mild to severe. The most common form of
color vision deficiency is red-green. Less common is
blue-yellow color vision deficiency.
Table 4: Prevalence of Inherited Color Vision
Deficiency in Children 61 Months (5 years) to 72
Months (6 Years) of Age
Color Vision
Deficiency
White Non-
Hispanic
Hispanic
African
American
Asian
Boys 7.8% 2.9% 2.1% 3.5%
Girls <0.4% <0.4% <0.4% <0.4%
Source: Multi-Ethnic Pediatric Eye Disease Study
69
• Ocular Diseases
Ocular inflammatory disease
Ocular inflammation in children involves an array of
conditions, including but not limited to conjunctivitis,
keratitis, scleritis, and uveitis. It may occur due
to infection, trauma, malignancy, or autoimmune
response. Inflammations can range from benign and
self-limiting to chronic and sight-threatening.
70, 71
Systemic autoimmune diseases in children can have
ocular manifestations that are vision-threatening.
Juvenile idiopathic arthritis is associated with the
development of chronic anterior uveitis. Other
diseases with ocular inflammatory manifestations
include sarcoidosis, juvenile rheumatoid arthritis,
Behçet’s disease, and Sjögren’s syndrome.
71, 72
Ocular conditions of prematurity
Children born prematurely are at risk for the
development of severe visual impairment and
blindness. Preterm infants have higher rates of

14
amblyopia, strabismus, optic atrophy, and refractive
errors.
73-76
Sixty percent of infants born at 28 to 31 weeks have
been reported to develop retinopathy of prematurity
(ROP) and over 80% of infants born before 28
weeks developed ROP.
77
ROP is also common in
children with birth weight of less than 1,251 grams
(g). Oxygenation of infants in the hours and days
after birth may also be a contributing factor.
78
The
frequency and severity of ROP is inversely related to
gestational age and birth weight of the baby.
79
The
incidence of ROP is 47% in infants with birth weights
between 1,000 and 1,251 g and 81.6% in infants
weighing <1,000 g at birth.
77
Cataract
Childhood cataracts can be classified as congenital
or developmental. They may be idiopathic, due
to infection (e.g., rubella), genetics (e.g., Down
syndrome), or the result of secondary causes such
as trauma or metabolic etiology. The prevalence of
visually significant congenital cataracts is estimated
to be three to four infants per 10,000 live births.
80
If not treated early, visually significant congenital
cataracts may cause vision impairment.
Glaucoma
Childhood glaucoma is an uncommon disease
characterized by increased intraocular pressure
leading to optic neuropathy and visual field changes,
and is often associated with significant vision loss.
81
It may be inherited or associated with other eye
disorders.
Glaucoma in children may be classified as congenital
(present at birth), infantile (occurring between 1 to
2 years of age), or juvenile (developing after age 3).
Most cases develop during the first year of life. A
review of records of pediatric patients seen in one
county in the United States over a 40-year period
found an incidence of glaucoma of 2.29 per 100,000
persons younger than 20 years of age.
81
Retinitis pigmentosa
Retinitis pigmentosa (RP) is a group of hereditary
retinal diseases characterized by progressive loss
of peripheral vision and the development of night
blindness. RP is caused by the degeneration of
photoreceptor cells resulting in severe damage to
the retina. While RP is usually limited to the eye, it
may also occur as part of a syndrome (e.g., Usher
syndrome, Bardet-Biedl syndrome).
82
Retinitis pigmentosa is the most frequent cause
of inherited visual impairment.
82
It is estimated to
affect 1 in 3,000 to 1 in 4,000 people in the United
States.
83
Retinoblastoma
Retinoblastoma, a cancer of the retina, usually
affects children under age 5. The most common
signs of retinoblastoma are leukocoria (white
pupillary reflex) and strabismus. Retinoblastoma
accounts for approximately 11% of cancers
occurring in the first year of life, with 95% diagnosed
before 5 years of age.
84
It is the most common
intraocular cancer of childhood and affects
approximately 300 children in the United States each
year. More than 90% of children with retinoblastoma
could be treated with early diagnosis;
85
however,
significant disparities exist in the care and outcomes
of children with retinoblastoma.
86
Retinoblastoma is associated with a mutation
of the RB1 gene. The tumor may be unilateral
or bilateral and can be inherited. Prognosis for
survival, saving the eye, and preservation of vision
are largely dependent on the stage of disease at
presentation. Early diagnosis, multidisciplinary
treatment, and genetic counseling are all priorities in
the management of this tumor.
87
Diabetic retinopathy
Diabetes is the third most common chronic disease
among children and a leading cause of vision
impairment among young adults. Type 1 diabetes
mellitus has historically been the most common

15
type in children, affecting approximately 2 per 1,000
school-age children in the United States; however,
Type 2 diabetes mellitus now accounts for about
45% of new cases of the disease.
88, 89
Diabetic retinal disease, primarily manifesting as
diabetic retinopathy (DR) and/or diabetic macular
edema, is the most common microvascular
complication of diabetes. Among pediatric patients,
the average duration of diabetes before the
development of DR is 5.7 to 9.1 years; however, the
risk for developing DR is greater in patients who are
diagnosed with diabetes during or after puberty.
88
Click to view the (AOA Clinical Practice Guidelines
web page)
Optic nerve hypoplasia
Optic nerve hypoplasia is one of the most prevalent
causes of visual impairment among young children.
Although the specific prevalence is unknown,
the Babies Count Registry reported optic nerve
hypoplasia as the third most prevalent cause of
vision impairment in children age 3 years or younger
in the United States.
90
The exact cause of optic nerve hypoplasia in not
known, but it may be associated with prenatal
exposure to alcohol, smoking, recreational drugs,
antidepressants and anticonvulsants, and with
prenatal complications including gestational
diabetes, toxemia, viral infection, and maternal
anemia. Seventy percent of the cases identified have
no known risk factors. More recent studies have
indicated the mother’s young age (≤ 20 years) and
primiparity (that is, the affected child is the mother’s
first child, regardless of the mother’s age) are the
predominant characteristics in the background of
children with optic nerve hypoplasia.
91
Optic nerve hypoplasia was believed to occur
either as an isolated anomaly or accompanying
the syndrome of septo-optic dysplasia or de
Morsier syndrome
92
that includes midline brain
malformations and hypopituitarism. Evidence now
suggests that optic nerve hypoplasia infrequently
occurs in isolation and is more appropriately
designated as the syndrome of optic nerve
hypoplasia.
93
In the syndrome, most children
with optic nerve hypoplasia have hypothalamic
dysfunction and/or neurodevelopmental impairment,
such as cerebral palsy or growth problems.
Cortical (cerebral) visual impairment
Cortical visual impairment (CVI) is defined as a
reduction or complete loss of visual acuity and
optokinetic nystagmus due to injury to the visual
cortex, with preservation of pupillary response,
normal eye motility, and normal retina.
94
In addition
to cortical visual impairment, the term cerebral visual
impairment is also used to describe not only visual
impairment associated with the visual cortex, but
also regions outside the cortex that can affect other
visual pathway structures.
95
In children experiencing perinatal or postnatal
hypoxia/ischemia, CVI, ROP, and optic nerve
hypoplasia were commonly identified conditions.
Of the three, CVI was the most prevalent visual
condition identified and was often the last to be
diagnosed.
90
Vision loss associated with brain damage is reported
to be a significant cause of visual impairment
in young children. Identification of children with
suspected CVI requires neuroimaging to ascertain
the extent of the injury to specific regions in the
brain. Failure to do so will underestimate the level of
visual dysfunction and systemic disability.
96
C. ACCESS TO CARE
Although comprehensive pediatric eye and vision
examinations are essential for timely diagnosis and
treatment of eye disease and maintenance of good
vision, many children do not receive comprehensive
eye care. An estimated one in five preschool children
and one in four school-age children in the United States
has a vision problem; however, the Centers for Disease
Control and Prevention report that less than 15% of
preschoolers receive an eye examination by an eye care

16
professional and less than 22% receive some type of
vision screening.
97
A factor that may limit access to comprehensive eye and
vision examinations and treatment services is the false
sense of security that school screenings mistakenly give
to parents (false negative results). Other factors that limit
access include the absence of signs, symptoms, or a
family history of eye and vision problems,
98
or the inability
of parents/caregivers to afford needed services due to
lack of insurance coverage or limited family income.
99
Limited access may now be partially resolved because
comprehensive eye and vision examinations have
received increased attention from the Affordable Care
Act and other insurance programs reviewing essential
health benefits necessary for children.
D. COST OF EYE AND VISION DISORDERS
IN CHILDREN
Eye and vision disorders can impose a significant burden
on patients, parents, and the public. The total economic
cost of vision loss and eye disorders among children
younger than 18 years of age in 2012 was estimated
to be $5.9 billion.
100
This includes the direct medical
costs for eye examinations, eyeglasses, and low vision
aids. Also, the debilitating nature of vision loss results in
major indirect and nonmedical costs including special
education services, federal assistance programs, and
decreased quality of life.
The above estimate does not include the costs of
educational services for children with undiagnosed
and untreated vision conditions. Learning-related
vision problems have been reported to be significant
contributors to reading difficulties and ultimately to the
need for special education services.
14, 15, 65, 101, 102
Vision
problems can increase educational costs in the form of
Individualized Education Programs (IEPs) and special
education services, which would otherwise not be
necessary, if the vision problems were treated. A study
of students (ages 6-16) with IEPs found that they have
high rates of undiagnosed and untreated vision problems
affecting reading speed and comprehension.
103
In addition to the current costs of care, future costs for
undiagnosed and untreated vision problems may include
the loss of a child’s full potential, and limitations on his
or her occupational choices and future earnings. The
cost of treating any visual impairment later in life could
potentially be more expensive than treatment of the initial
problem.
E. EARLY DETECTION AND PREVENTION
OF EYE AND VISION DISORDERS
Many vision conditions are asymptomatic or not readily
recognized, and will not prompt a patient, caregiver,
or parent to seek a comprehensive eye and vision
examination.
104
Undiagnosed or uncorrected refractive
errors and other visual disorders in children can lead
to developmental, academic, and social challenges
and in some cases permanent vision loss, which has
lifelong complications.
105
In the preschool population, the
concern is for early diagnosis and treatment of significant
refractive error, amblyopia, strabismus, and ocular
disease. For the school-age population, the concern
is the negative impact that untreated vision disorders
(accommodation, binocular vision, ocular motility,
and vision information processing) have on academic
performance.
A comprehensive eye examination by a doctor of
optometry or ophthalmologist is the reference standard
of eye care.
105
Not all children receive professional eye
examinations for various reasons including education
and language barriers, health literacy, cost, geographic
access, immigration status, and transportation
challenges.
106
The role of vision screenings in addressing the current
gaps in children’s eye care remains unclear. The U.S.
Preventive Services Task Force (USPSTF) has concluded
that the current evidence is insufficient to assess the
balance of benefits and harms of vision screening
for children 3 years of age and younger.
107
While the
USPSTF concluded with moderate certainty that vision
screening for children 3 to 5 years of age has moderate
net benefit compared with no screening, they did not
compare the benefit of screening to a comprehensive
eye examination.
108
Vision screening procedures lack the evidence needed,
with proven high sensitivity and specificity, for identifying

17
the targeted vision problems present in the population
of children being screened.
104,109
The sensitivity of a
wide variety of screening techniques was evaluated
by the Vision in Preschoolers (VIP) study, which unlike
standard screenings used licensed eye doctors who had
completed VIP study specific training and certification.
104
In the study, the sensitivity of 11 vision screening
techniques used for detecting clinically significant vision
problems in children 3 to 5 years of age varied from 16%
to 64%, with specificities ranging from 62% to 98%.
These tests were compared again with a specificity of
94%, and the sensitivity dropped even further.
109
When
these same tests were performed by trained nurses or
lay screeners (except for non-cycloplegic retinoscopy,
which was deemed too technically challenging), the
sensitivity was similar or lower.
110
Even with the use of
trained examiners, these vision screening techniques
were unable to provide high levels of both sensitivity and
specificity for detecting many vision problems in children.
Currently, widespread application of vision screenings
do not utilize licensed eye care professionals or the
techniques found to be most sensitive.
When Snellen visual acuity alone was used as a
screening tool, it was 100% specific for identifying
reduced acuity, but missed 75.5% of the children found
to have binocular and oculomotor vision problems when
given a complete visual examination.
111
Additionally, a
study of 1,992 school-age children found that 41% of
children who failed the State University of New York
screening battery would not have been identified if the
screening was based on visual acuity alone.
112
Many children who fail a screening do not receive the
necessary treatment of their conditions. A study of public
school children reported only 38.7% who failed the vision
screening received follow-up care after screenings.
113
Due to a lack of follow-through, screenings alone may
not lead to the earlier diagnosis and treatment of eye
and vision problems. While screenings may identify some
children at risk for vision problems, a comprehensive
eye exam is necessary for definitive diagnosis and
appropriate treatment.
114
III. CARE PROCESS
A. COMPREHENSIVE PEDIATRIC EYE AND
VISION EXAMINATION
The comprehensive pediatric eye and vision examination
provides the means to evaluate the structure, function,
and health of the eyes and visual system. It is preferable
in most cases for the parent/caregiver to accompany
the child into the examination room. The in-person
interaction between patient/parent/caregiver and doctor
is a dynamic process. It involves collecting subjective
data from the patient/parent/caregiver and obtaining
objective data by observation, examination, and testing.
During the examination, information is obtained to
explain symptoms reported by the patient and/or parent/
caregiver and diagnose their cause. It also provides
the means to identify the presence of other ocular or
systemic conditions that may exist with or without
symptoms. (See Appendix Figure 1.)
The goals of the comprehensive pediatric eye and vision
examination are to:
• Evaluate the refractive, binocular, and
accommodative status of the eyes and visual
system, taking into account special vision demands
and needs
• Assess ocular health and related systemic health
conditions
• Establish a diagnosis (or diagnoses)
• Formulate a treatment and management plan
• Counsel and educate the patient/parent/caregiver
regarding visual, ocular, and related systemic
health care status, including recommendations for
prevention, treatment, management, and future care.
1. General Considerations
Since the capabilities and needs of children vary
significantly by age, the potential components of the
comprehensive pediatric eye and vision examination
have been divided into three age groups. This
subdivision of the pediatric population is based on the
developmental changes that occur from birth through
childhood. The following age groups were also chosen
18
to be compatible with those used by other medical and
governmental groups involved with children’s health.
Because an individual child’s development can vary
significantly from expected age norms, it is important
not to rely solely upon chronological age when choosing
testing procedures. Appropriate test procedures need to
be based on the child’s developmental age and specific
capability.
a. Infants and Toddlers (newborn through 2 years of
age)
Children in this age group may perform best if the
examination is early in the morning or after an infant’s
nap. Age-appropriate examination strategies should be
used. It is necessary to rely on objective examination
procedures and to perform tests more rapidly than with
older children.
b. Preschool Children (3 years through 5 years of
age)
At about 3 years of age, children have achieved
adequate receptive and expressive language skills
to begin to cooperate for some of the traditional eye
and vision tests; however, testing modifications are
often needed to gather useful information. Beginning
the examination with procedures that appear less
threatening may help to put the child at ease. The use of
subjective tests requiring verbal interaction may need to
be modified.
c. School-age Children (6 through 18 years of age)
Most of the examination procedures used on
adults apply to this age group; however, for some
patients, modifications should be made to improve
understanding and cooperation. Utilization of tests
designed for younger age groups may be appropriate.
Tests of accommodation, oculomotor skills, and
binocular function should be included as part of the
comprehensive examination.
2. Examination Procedures**
The examination procedures described are not intended
to be all-inclusive. Professional judgment and individual
patient symptoms and findings may significantly influence
the nature and course of the examination. It is important
to remain alert for new and emerging technologies,
instruments, and procedures and incorporate them into
the clinical examination, as appropriate.
CONSENSUS-BASED ACTION STATEMENT:
A comprehensive pediatric eye and vision
examination should include, but is not limited to:
• Review of the nature and history of the presenting
problem, patient and family eye and medical
histories, including visual, ocular, general health,
leisure and sports activities, and developmental
and school performance history of the child
• Measurement of visual acuity
• Determination of refractive status
• Assessment of binocular vision, ocular motility, and
accommodation
• Evaluation of color vision (baseline or periodic, if
needed, for qualification purposes or if disease
related)
• Assessment of ocular and systemic health,
including evaluation of pupillary responses,
anterior and posterior segment, peripheral retina,
evaluation/measurement of intraocular pressure,
and visual field testing.
Refer to section III. Care Process, A. 9 for a listing of
potential benefits and harms of testing.
Evidence Quality: There is a lack of published
research to support or refute the use of all of
the tests and/or assessments included in this
recommendation.
Benefit and Harm Assessment: Implementation
of this recommendation is likely to result in the
enhanced ability to diagnose any eye or vision
problems in infants and children. The benefits of
this recommendation were established by expert
consensus opinion.
** See Appendix Tables 1, 2, and 3 for a listing of
specific tests by age group.

19
3. Patient History
The patient history is an initial and ongoing component
of the examination. The objective is to obtain specific
information about the patient and/or parent’s/caregiver’s
perception of the child’s eye and vision status and
important background information on related medical
issues. It helps to identify and assess problems, and it
provides an opportunity to become acquainted with the
patient and/or his/her parents or caregivers, establishing
a relationship of confidence and trust.
The collection of demographic data generally precedes
the taking of the patient history. Having the parent
or caregiver fill out a questionnaire may facilitate
obtaining the patient and family history, if known. Major
components of the patient history include, but are not
limited to:
• Nature and history of the presenting problem,
including chief complaint
• Visual and ocular history
• General health history, including prenatal,
perinatal and postnatal history, and review of
systems, surgical and/or head or ocular trauma
history, and any vision or ocular treatment
• Medication reconciliation, including prescription
and nonprescription drugs (e.g., over the
counter medications, supplements, herbal
remedies) and documentation of medication
allergies
• Family ocular and medical history
• Clinical note: It is recommended that the
patient history should also include the refractive
status of both parents,
115, 116
(Evidence Grade:
B) because it is a possible risk factor for the
progression of myopia in school-age children.
• Developmental history of the child
• School performance history of school-age
children
• Time spent outdoors, on sports activities, and
on near work and screen viewing
• Names of, and contact information for, the
patient’s other health care providers.
4. Testing
4.1 Testing of Infants and Toddlers (newborn
through 2 years of age)
a. Visual Acuity
Estimation of visual acuity in an infant or toddler can help
to confirm or reject certain hypotheses about the level of
visual system development, including binocularity, and
provide direction for the remainder of the eye and vision
examination. Assessment of visual acuity for infants and
toddlers may include these procedures:
• Preferential looking visual acuity
Preferential looking methods are useful for the
assessment of visual acuity in infants and toddlers.
Grating acuity targets (e.g., Teller acuity cards) and
vanishing optotypes (e.g., Cardiff acuity test) can
provide estimates of resolution visual acuity.
117
• Fixation preference test
Fixation preference testing results need to be
interpreted in the context of all other available
information (e.g., degree and type of anisometropia,
frequency and type of strabismus). Results of
fixation preference testing may be unreliable for
diagnosing amblyopia,
118, 119
particularly secondary
to anisometropia; therefore, monocular visual acuity
measurements should be obtained, if possible.
120
• Visual evoked potential
Electrodiagnostic testing, such as visual evoked
potentials, is an objective method that can be used
to provide an estimate of visual acuity in infants.
121
b. Refraction
Objective measures of refraction with a lens bar or loose
lenses should be used in this age group because of the
short attention span and poor fixation of infants. The
refractive error measurement should be analyzed with
other testing data obtained during the examination. This
20
information is used to determine if, and in what amount,
an optical correction is needed. Procedures may include:
• Cycloplegic retinoscopy
When performing cycloplegic retinoscopy in an infant
or toddler, the appropriate cycloplegic agent should
be selected carefully.
122
The lowest concentration
of drug that yields the desired cycloplegia should
be used. A concentration of 0.5% cyclopentolate
hydrochloride can be used in most infants under
12 months of age and a 1% concentration for
older children.
123
Combination drops (0.2%
cyclopentolate hydrochloride and 1% phenylephrine)
are also available for use with infants. The potential
for systemic absorption may be reduced with
nasolacrimal occlusion. The cycloplegic of choice
is cyclopentolate hydrochloride; however, when it
is not available or is contraindicated, tropicamide
1% has also been shown to be effective for the
measurement of refractive error in non-strabismic
infants.
124
Spray administration of cyclopentolate to the open
or closed eyes of young children is an acceptable
alternative, if necessary, to using eye drops and is
often better tolerated and less distressing than other
methods of drug administration;
125-128
however, the
use of cyclopentolate spray in children with dark
irides may not achieve adequate cycloplegia.
129
Spray caps are available for use on bottles of
cyclopentolate, eliminating the need to have the
spray compounded by a pharmacy.
• Non-cycloplegic retinoscopy
Non-cycloplegic retinoscopy performed at near is
an objective means of estimating refractive error
in infants and toddlers,
130
but should be used with
caution as a substitute for cycloplegic retinoscopy.
131
It may be useful when a child/parent is extremely
anxious about instillation of cycloplegic agents,
or the child has had, or is at risk for, an adverse
reaction to cycloplegic agents.
132
Video refraction without cycloplegia can also be
used to detect infants with significant ametropia,
particularly hyperopia or other accommodative
problems.
133
c. Binocular Vision and Ocular Motility
Depending on the patient’s age, level of cooperation,
and visual signs and symptoms, appropriate tests
of binocular vision and ocular motility should be
incorporated into the examination. Testing in this age
group may include:
• Ocular alignment assessment
The unilateral cover test at distance and near can
generally be used with very young children. If cover
test results are unreliable because of the child’s
resistance to testing, use of the Hirschberg test
may be successful. Prisms can be used with the
Hirschberg test to align the corneal reflex (Krimsky
test) and estimate the magnitude of any deviation.
• Brückner test
If cover test results are equivocal, particularly in
young or uncooperative patients, the Brückner test
may be helpful in detecting strabismus, including
small angle strabismus. It may also be useful in
the clinical evaluation of anisometropia in infants
and young children.
134
Increasing the examination
distance from one meter to four meters can improve
its sensitivity for detecting anisometropia.
135
• Stereopsis
Testing of stereopsis, after 6 months of age, can
provide a sensitive measure of visual development in
infants.
136
In this population, tests like the Preschool
Assessment of Stereopsis with a Smile (PASS) 3,
which uses a preferential looking paradigm, should
be used.
• Near point of convergence (NPC)
Assessment of convergence ability may be
determined objectively in infants using a penlight or
other interesting targets, which include sounds or
blinking lights.

21
• Ocular motility assessment
Versions and eye tracking abilities may be assessed
using a penlight, small toy, or other object.
4.2 Testing of Preschool Children (3 through 5 years
of age)
a. Visual Acuity
The accurate measurement of visual acuity in children
allows for the early detection of amblyopia and
significant/high refractive errors. While some children in
this age group may respond verbally, acuity testing may
require the use of a matching or a forced-choice task. An
assessment of visual acuity may include the use of:
• Symbol optotype or letter matching visual acuity
measurement
Symbol optotype or letter optotype testing (e.g., Lea
symbols) and letter matching testing (e.g., HOTV)
can be used to measure the visual acuity of most
children aged 3 through 5 years.
137-140
b. Refraction
A refraction should include objective and, as appropriate,
subjective assessment of the child’s refractive status;
however, the results of a refraction do not provide
all the information needed to determine an optical
prescription. The refractive error measurement should be
analyzed with other testing data and the patient’s visual
needs obtained during the in-person examination. This
information is used to determine if, and in what amount,
an optical correction is needed to provide optimal vision
and comfort for all viewing distances. Testing in this age
group may include:
• Static (distance) retinoscopy
Use of a lens rack or loose lenses with appropriate
control of accommodation, rather than a phoropter,
enables the child’s eyes to be seen and allows for
observation if the child loses fixation. Viewing a video
may assist in capturing a child’s attention in order to
sustain distance fixation.
• Cycloplegic retinoscopy
Spray administration of cyclopentolate to the open
or closed eyes of young children is an acceptable
alternative, if necessary, to using eye drops and is
often better tolerated and less distressing than other
methods of drug administration;
125-128
however, the
use of cyclopentolate spray in children with dark
irides may not achieve adequate cyclopelgia.
129
Spray caps are available for use on bottles of
cyclopentolate, eliminating the need to have the
spray compounded by a pharmacy.
CONSENSUS-BASED ACTION STATEMENT:
Cycloplegic retinoscopy is the preferred procedure
for the first evaluation of preschoolers. It is
necessary to quantify significant refractive error
in the presence of visual conditions such as
strabismus, amblyopia, and anisometropia.
Evidence Quality: There is a lack of published
research to support or refute the use of this
recommendation.
Benefit and Harm Assessment: Implementation
of this recommendation is likely to enhance the
ability to evaluate and diagnose eye and vision
problems in preschool children. The benefits of
this recommendation were established by expert
consensus opinion.
• Autorefraction
The use of a hand-held autorefractor is preferable in
this age group since it may be less intimidating than
a table mounted instrument.
Autorefractors can provide an objective measure
of refractive error, but may underestimate the level
of hyperopia and overestimate the level of myopia
under non-cycloplegic conditions,
141, 142
and their
usefulness in testing children less than 3 years of
age may be limited.
143

22
c. Binocular Vision, Ocular Motility, and
Accommodation
• Ocular alignment assessment (distance and
near)
Testing should include use of the unilateral cover
test and alternating cover test. If cover test results
are unreliable because of the child’s resistance
to testing, use of the Hirschberg test may be
successful. Prisms can be used with the Hirschberg
test to align the corneal reflex (Krimsky test) and
estimate the magnitude of any deviation.
• Ocular motility assessment
Examination of eye movements in this age group
involves an assessment of comitancy.
• Near point of convergence (NPC)
Assessment of maximum convergence ability may
be determined objectively or subjectively.
• Stereopsis
In the preschool population, stereopsis testing can
provide useful information about development of
binocular vision and eye alignment. Testability in this
age group has been reported to be close to 90%
using age appropriate techniques.
136, 144, 145
The
presence of global stereopsis is an indication that
the patient is bifoveally fixating and evidence that a
constant strabismus is less likely to be present.
146,
147
This information is valuable when the cover test
results are equivocal and the clinician suspects a
small angle, constant strabismus may be present.
To accomplish this objective, a stereopsis test that
assesses global, rather than local stereopsis, should
be used. The PASS 3 and the Randot Preschool
tests are examples of global stereopsis tests that
can be used for this purpose. Stereopsis tests that
have monocular cues (local stereopsis e.g., Titmus
Test) may lead to false-positive results.
147
• Positive and negative fusional vergence ranges
Assessment of positive and negative fusional
vergence ranges can be done using a step vergence
procedure with a hand-held prism bar.
37, 148
• Accommodative testing
Clinical note: Dynamic retinoscopy has been
shown to be a reliable method for assessing
accommodation in young children.
149,150
(Evidence
Grade: B)
d. Color Vision
Children with color vision deficiency, either congenital or
acquired, may have difficulty precisely matching colors
or discriminating fine color differences.
151
The severity
of color vision deficiency can range from mild to severe
depending on the cause. Most children can be reliably
evaluated for color vision deficiency after 60 months (5
years) of age.
69
It is helpful to know whether a color vision deficiency
exists, because severe color vision deficiency may
cause a child to be misidentified as learning disabled.
152
Identification of abnormal color vision prior to school
age is also important, since part of the early educational
process generally involves the use of color identification
and discrimination. The presence of a color vision
deficiency may also indicate an ocular health problem;
therefore, color vision testing may need to be repeated, if
an acquired color vision deficiency is suspected.
Although effective when used with standard illuminant,
some pseudoisochromatic plate tests only detect protan
and deutan color vision deficiency,
153
while other color
vision tests provide the added advantage of detection
of tritan defects and the ability to categorize defects as
mild, moderate, or severe.
154
4.3 Testing of School-age Children (6 through 18
years of age)
a. Visual Acuity
Visual acuity may be measured monocularly and
binocularly, at distance and near, with and without the
child’s most recent spectacle or contact lens correction.

23
An assessment of visual acuity in children age 6 years or
older may include:
• Snellen visual acuity
For some children, Snellen visual acuity testing may
need to be modified by isolating one line, or even
one-half line of letters. If amblyopia is suspected,
single letters with surround bars should be used.
• Early Treatment of Diabetic Retinopathy Study
(ETDRS) visual acuity chart
The ETDRS chart may be used to measure visual
acuity in school-age children
155
and can be especially
useful in diagnosing and monitoring children with
amblyopia.
b. Refraction
A refraction may include objective and subjective
assessment of a child’s refractive status; however, the
results of a refraction do not provide all the information
needed to determine an optical prescription. The
refractive error measurement should be analyzed with
other testing data and the patient’s visual needs obtained
during the in-person examination. This information is
used to determine if, and in what amount, an optical
correction is needed to provide optimal vision and
comfort for all viewing distances.
Both objective and subjective testing for refractive error
can generally be used in this age group. It may include:
• Static (distance) retinoscopy
Retinoscopy may be performed with a phoropter, or
without a phoropter using a lens rack or loose lenses
and fogging glasses.
• Cycloplegic retinoscopy
CONSENSUS-BASED ACTION STATEMENT:
Cycloplegic retinoscopy is the preferred procedure
for the first evaluation of school-age children.
It is necessary to quantify significant refractive
error in the presence of visual conditions such as
strabismus, amblyopia, and anisometropia.
Evidence Quality: There is a lack of published
research to support or refute the use of this
recommendation.
Benefit and Harm Assessment: Implementation
of this recommendation is likely to enhance the
ability to evaluate and diagnose eye and vision
problems in school-age children. The benefits of
this recommendation were established by expert
consensus opinion.
Clinical note: In school-age children, cycloplegic
refraction results in a more positive spherical
power measurement than that obtained
using optical fogging techniques to relax
accommodation.
156
(Evidence Grade: B) The
difference in spherical equivalent refractive errors
measured in pre- and post-cycloplegic refractions
is significant up until age 20.
157
(Evidence Grade:
B)
• Subjective refraction
Typical examination procedures used to measure
refractive error in adults can generally be used for
school-age children.
• Autorefraction
Autorefraction may be used as a starting point
for subjective refraction, but not as a substitute
for it; however, retinoscopy, when performed by
an experienced clinician, is more accurate than
automated refraction for determining a starting point
for non-cycloplegic refraction.
158
(Evidence Grade: C)
c. Binocular Vision, Ocular Motility, and
Accommodation
In analyzing the results of these tests, it is important
to examine all the data and group findings rather than
depend on a single finding to arrive at a diagnosis.
Testing in this age group is similar to that for adults and
may include:

24
• Ocular alignment assessment (distance and
near)
Testing may use the unilateral cover test and
alternating cover test. If cover test results are
unreliable because of the child’s resistance
to testing, use of the Hirschberg test may be
successful. Prisms can be used with the Hirschberg
test to align the corneal reflex (Krimsky test) and
estimate the magnitude of any deviation. Other tests
include the Von Graefe phoria, Modified Thorington,
and Maddox Rod.
• Ocular motility assessment
Examination of eye movements in this age group
involves an assessment of comitancy of fixation,
saccadic, and pursuit functions. Versions may also
be performed to rule out a noncomitant deviation.
• Near point of convergence (NPC)
Determination of maximum convergence ability may
be obtained objectively or subjectively.
• Stereopsis
School-age children should be able to complete
any of the available random dot stereopsis
tests. If random dot (global) stereopsis is not
present, testing should continue to evaluate local
stereopsis, potential for flat fusion, and potential for
simultaneous perception.
• Positive and negative fusional vergence ranges
Evaluation should be made of both the amplitude
and facility of fusional vergence ranges.
• Accommodative testing
Assessment of accommodation may include
accommodative amplitude, facility, and
response.
149,159
Testing of negative relative
accommodation (NRA) and positive relative
accommodation (PRA) may provide useful
information on both accommodative and binocular
status.
149
d. Color Vision
If not done previously, school-age children should be
tested for color vision deficiency. Color vision deficiency
can interfere with daily activities involving colors and
prohibit some occupational choices.
160
One-third
of individuals with abnormal color vision reported
their career choice had been affected by color vision
deficiency and one-quarter had been precluded from an
occupation because of it or had problems in their current
job.
161
CONSENSUS-BASED ACTION STATEMENT:
Abnormal color vision can affect daily performance
of activities involving color discrimination and
may interfere with or prevent some occupational
choices later in life. Children should be tested as
soon as possible for color vision deficiency and the
parents/caregivers of children identified with color
vision deficiency should be counseled.
Evidence Quality: There is a lack of published
research to support or refute the use of this
recommendation.
Benefit and Harm Assessment: Implementation
of this recommendation is likely to increase early
detection of color vision deficiency and alert
parents/caregivers to any potential effects on a
child’s education or occupational choices. The
benefits of this recommendation were established
by expert consensus opinion.
5. Ocular and Systemic Health Assessment
Thorough assessment of the health of the eyes and
associated structures is an important and integral
component of the comprehensive pediatric eye and
vision examination. The eyes and associated structures
are not only sites for primary ocular diseases, but they
are also subject to systemic disease processes that
affect the body as a whole (e.g., disorders of neurologic,
vascular, endocrine, immune, or neoplastic origin).
Standard procedures used in evaluating adult patients
may need to be modified or may not be optimal
in very young patients. With some modifications,

25
the components of the ocular and systemic health
assessment may include:
a. Assessment of Pupillary Responses
Evaluation of pupils includes size, shape, symmetry,
and direct and consensual response to light and relative
afferent pupillary defect.
b. Visual Field Evaluation
Confrontation visual field testing can be used to detect
gross peripheral defects and areas of constricted visual
fields.
c. Evaluation of the Ocular Anterior Segment and
Adnexa
Assessment of the external eye and adnexa, ocular
surface, anterior chamber, and crystalline lens.
d. Evaluation of the Ocular Posterior Segment
Pharmacological dilation of the pupil is generally required
for thorough stereoscopic evaluation of the ocular
media, retinal vasculature, macula, optic nerve, and the
peripheral retina.
162
(Evidence Grade: B)
Examination under general anesthesia may be
considered under rare circumstances, if the retina cannot
be adequately visualized during an examination of at-risk
children.
163
e. Measurement of Intraocular Pressure
Measuring intraocular pressure (IOP) is a part of the
comprehensive pediatric eye and vision examination.
Although the prevalence of glaucoma is low in children,
measurement of IOP should be attempted. Pressure
should be assessed when ocular signs and symptoms or
risk factors for glaucoma exist. If risk factors are present
and reliable assessment of IOP under standard clinical
conditions is impossible, testing under anesthesia may
be indicated. Recording of tonometry results should
include method used and time of day.
164
(Evidence
Grade: C)
Clinical note: The Goldmann applanation
tonometer (GAT) is considered the reference
standard for the measurement of IOP; however,
its use may not be practical in very young
children. Non-contact and handheld applanation
tonometers can provide IOP measurements close
to that of the Goldmann.
165
(Evidence Grade: A)
Rebound tonometry offers an advantage over
GAT in that it is portable, easy to use, and better
tolerated.
166
(Evidence Grade: B)
6. Supplemental Testing
During an eye and vision examination, the information
obtained from the patient is continually assessed, along
with the clinical findings gathered. The interpretation of
subjective and objective data may indicate the need for
additional testing.
Additional testing may be indicated to:
• Confirm or rule out differential diagnoses
• Enable more in-depth assessment
• Provide alternative means of evaluating patients
who may not be fully cooperative or who may
not comprehend testing procedures.
Supplemental procedures may be performed
immediately or during subsequent examinations.
Supplemental testing for infants and children may
include:
a. Electrodiagnostic Testing
Electrophysiological techniques may be used to assess
children with unexplained reduced vision. Testing may
include an electroretinogram (ERG) or measurement of
visual evoked potential (VEP).
b. Imaging
The following procedures may be used for imaging of
ocular structures:

26
• Ultrasonography can reveal congenital
anatomical abnormalities in the eye and orbit,
as well as anatomical changes secondary to
disease or injury, and measure axial length
• Optical coherence tomography (OCT) provides
cross-sectional, high-resolution imaging of the
anterior and posterior segments
• Scanning laser ophthalmoscopy provides 3-D
images of the optic nerve head
• Fundus photography, with or without auto
fluorescence, is a noninvasive diagnostic
technique for examining the fundus
• Corneal topography provides an assessment of
corneal thickness, shape, power, and surface
details
• Computerized tomography (CT) scan,
magnetic resonance imaging (MRI), and other
neuroimaging may be indicated for suspicion of
neurological disease or trauma/injury
• Scheimpflug camera for anterior segment
tomography (Pentacam, Orbscan, and Gallilei)
may be used for detection of keratoconus.
c. Testing for Learning-related Vision Problems
Vision problems such as accommodative, binocular
vision, eye movement, and visual information processing
disorders can interfere with academic performance.
When a child’s history or initial testing indicates a
possible developmental lag or learning disorder,
additional testing should be performed to rule out a
learning-related vision disorder. This will typically require
an additional office visit that includes more extensive
testing of accommodation, binocular vision, and eye
movements, and an assessment of visual information
processing skills. In some instances, this may require a
referral to a doctor of optometry with advanced training
in this area of practice.
CONSENSUS-BASED ACTION STATEMENT:
Children at risk for learning-related vision problems
should be evaluated by a doctor of optometry.
Evidence Quality: There is a lack of published
research to support or refute the use of this
recommendation.
Benefit and Harm Assessment: Implementation
of this recommendation is likely to result in more
in-depth evaluation and diagnosis of children with
learning-related vision problems. The benefits of
this recommendation were established by expert
consensus opinion.
Click to view the (AOA Clinical Practice Guidelines
web page)
7. Children with Special Needs
a. At-risk Children
In the United States, the Individuals with Disabilities
Education Act (IDEA) allows for the development of
Individualized Education Programs (IEPs) when indicated.
The following categories of children are considered high-
risk (Health and Human Services, Health Resources
and Services Administration, Maternal and Child
Health Bureau) and recommend direct referral for a
comprehensive eye and vision examination:
• Children with obvious evidence of physical
anomaly (e.g., strabismus, ptosis, nystagmus)
• Children with central nervous system (CNS)
dysfunction (e.g., cerebral palsy, Down
syndrome, seizures, developmental delay)
• Children with Autism Spectrum Disorder
• Children enrolled in Early Intervention (EI)
Program’s
a). Children with an IEP
b). Children enrolled in Early Head Start (ages
0-3)
• Children with a family history of amblyopia,
strabismus, or other early eye disease

27
• Children born from high-risk pregnancy (e.g.,
maternal drug use, infection during pregnancy,
preterm delivery).
b. Developmental Disabilities
Many children with special needs have undetected
and untreated visual problems
167
(see Appendix
Table 4: Partial Listing of Ocular Manifestations of
Neurodevelopmental Disorders and Other Syndromes).
Children with developmental or intellectual disabilities
have a higher rate of vision disorders and should
receive a comprehensive pediatric eye and vision
examination.
21, 25, 168
Although clinically more challenging,
visual assessment is possible in the majority of these
children.
167
(Evidence Grade: B),
169
(Evidence Grade:
B) Early identification of specific visual deficits could
lead to interventions to improve the educational and
occupational achievement and quality of life for these
high-risk children.
CONSENSUS-BASED ACTION STATEMENT:
Many children with developmental or intellectual
disabilities have undetected and untreated vision
problems and should receive a comprehensive
pediatric eye and vision examination.
Evidence Quality: There is a lack of published
research to support or refute the use of this
recommendation.
Benefit and Harm Assessment: Implementation
of this recommendation is likely to result in
improved quality of life and educational and
occupational achievement for these high-risk
children. The benefits of this recommendation
were established by expert consensus opinion.
8. Trauma and Ocular Manifestations of Child
Abuse/Neglect
a. Trauma (Accidental)
A majority of concussions occur in the pediatric and
adolescent population (5 to 17 years of age), with
the 11 to 17-year-old group representing the largest
proportion of those injured.
170, 171
Children are particularly
vulnerable to the consequences of concussion, often
having a more prolonged recovery and poorer outcomes,
from a cognitive and developmental perspective, than
adults with concussion.
172-175
A recent study found
a high prevalence of vision problems in adolescents
with concussion along with significant symptoms
associated with these vision disorders.
176
The most
common binocular vision disorder occurring in post-
concussion syndrome is convergence insufficiency (CI)
with a prevalence of 49% in children. Other common
problems are accommodative insufficiency and saccadic
dysfunction.
All children with concussion should see their general
practitioner in the event they should need more emergent
care and should be scheduled for a comprehensive eye
examination to confirm visual capabilities are protected.
b. Ocular Manifestations of Child Abuse and Neglect
(Non-accidental)
External eye trauma (e.g., conjunctival hemorrhages,
lid lacerations, corneal scars and/or opacities) and
retinal trauma (e.g., hemorrhages, folds, tears, and/
or detachments) are common ocular findings from
child abuse and can have an important role in its
diagnosis.
177-180
Most often the child is between 2 and 18
months of age at the time of abuse.
179, 181
The eyes can be direct or indirect targets of child abuse
and may provide valuable diagnostic information,
particularly when there are limited external signs
of abuse. In children, the leading cause of retinal
hemorrhages with retinal folds and macular retinoschisis,
in the absence of skull fractures or automobile accident
history, is typically abusive head trauma.
182, 183
Retinal
hemorrhages, poor visual response, and poor pupil
response in an infant may indicate abusive head trauma,
or Shaken Baby Syndrome,
177
(Evidence Grade: B),
178
(Evidence Grade: C) a form of child abuse in which the
child is injured secondary to violent shaking.
A vague history provided by the parent/caregiver that
changes on re-questioning or is inconsistent with the
age of the child or extent of the injury should be an alert
for abuse. In such cases, a detailed history is one of
the most important factors to consider when assessing
whether a child has been abused.
180

28
Table 5: Summary of the Signs of Child Abuse and Neglect
Ocular signs of abuse
General physical signs of abuse
or neglect
Emotional and behavioral signs
of abuse or neglect
Cortical blindness
Ruptured globe
Retinal, preretinal, vitreous hemorrhages
particularly if child is less than 2
years old
Detached retina, retinal dialysis
Chorioretinal atrophy
Papilledema
Optic atrophy
Cataracts
Dislocated, subluxated lens
Glaucoma
Shallow anterior angle
Angle recession
Iris tears, iris dialysis
Pupillary anomalies
Anisocoria
Hyphema
Hypopyon
Corneal scars, edema, opacities
Conjunctival, subconjunctival hemorrhages
Orbital, periorbital edema
Lid lacerations
Ptosis
Proptosis
Esotropia
Strabismus
Nystagmus
Disconjugate eye movements
Eyelash infestation with phthirus pubis
(crab louse)
Bruises around cheeks, jaw, eyes, ears, or
mastoid area
Soft tissue bruises on upper arms, thighs,
buttocks or genitals
Hair loss with/without subgaleal menatoma
Torn frenum of upper lip
Torn floor of mouth
Burns on any posterior part of the body,
particularly buttocks, perineum, hands,
or feet
Full thickness burns
Multiple lesions or fractures in different
stages of healing
Poor hygiene
Inferior general health
Signs of malnutrition such as sunken
cheeks and buttocks, distended
abdomen
Child not properly immunized
Venereal disease in a preadolescent child
Case history inconsistencies
No history offered
History vague or inconsistent with injuries
History changes during course of
examination
History varies between two parents or
between parents and child
Multiple office visits for accidental injuries
Increase in severity of injuries
Delay in seeking medical attention
Frozen watchfulness
Fear of strangers
Indiscriminate attachment to strangers
Failure to thrive
Growth failure
Low intellectual performance
Sad affect
Low self-esteem
Impaired ability to enjoy life
Social withdrawal
Learned helplessness
Suicidal ideation or attempts
Drug or alcohol abuse
Misconduct in school
Academic failure
Low school attendance
Aggressive behavior
Sleeping problems
Running away
Low level of activity
Weight fluctuation
Fatigue
Generalized anxiety
Sexual acting out
Source: Smith S. Child abuse and neglect: A diagnostic guide for the optometrist. J Amer Optom Assoc 1988;
59:760-66.
All 50 states and the District of Columbia have laws mandating the reporting of suspected child abuse and provide
penalties for failure to do so.
U.S. Department of Health and Human Services, Administration for Children & Families, Children’s Bureau listing of
state child abuse and neglect reporting numbers
Clinical note: Doctors of optometry should be aware of the eye-related findings associated with abusive head
trauma and report findings of possible child abuse to the proper authorities, as defined by state law, for the
protection of the child.

29
9. Potential Benefits and Harms of Testing
The potential benefits of a comprehensive pediatric eye
and vision examination include:
• Optimizing visual function through diagnosis,
treatment, and management of refractive, ocular
motor, accommodative, and binocular vision
problems
• Preventing and/or minimizing vision loss through
early diagnosis, treatment, and management of
ocular health conditions
• Detecting systemic disease and referring for
appropriate care
• Counseling and educating patients/parents/
caregivers on current conditions and preventive
care to maintain ocular and systemic health and
visual function, and on the relationship between
vision problems and early learning.
Potential harms associated with a comprehensive
pediatric eye and vision examination may include:
• Patient or parent/caregiver anxiety about testing
procedures or resulting diagnosis
• Adverse ocular and/or systemic reactions and/
or temporary visual disturbances resulting from
testing, or allergic responses to diagnostic
pharmaceutical agents or materials used
• Missed or misdiagnosis of eye health or vision
problems
• Unnecessary referral or treatment.
B. ASSESSMENT AND DIAGNOSIS
At the completion of the examination, the data collected
should be assessed and evaluated to establish a
diagnosis (or diagnoses) and formulate a treatment
and management plan. The nature and severity of the
problem(s) diagnosed determine the need for optical
prescription (e.g., eyeglasses and/or contact lenses) or
other treatment (e.g., vision rehabilitation, vision therapy,
ocular pharmaceuticals).
C. MANAGEMENT
1. Prescription for Correction
A prescription for correction of refractive error, if needed,
is provided at the conclusion of the examination.
184
The
level of refractive error may be monitored rather than
prescribed as a lens correction, or full or partial optical
correction may be prescribed, depending on the specific
visual needs, refractive measurement, and related visual
findings.
2. Additional Treatment Services
Depending on the diagnosed eye and vision condition(s),
other treatment services may be needed. For conditions
such as accommodative, binocular vision, eye
movement, visual information processing disorders, or
visual impairment, treatment such as the use of prisms,
vision therapy, or vision rehabilitation may be necessary.
Ocular pharmaceuticals may also be used for the
treatment of various eye diseases.
3. Counseling and Education
It is important for children/parents/caregivers to
understand the medical information and
recommendations given to them. To enhance
understanding, open-ended questions should be used.
Children/parents/caregivers should be asked to state
their understanding of the information given to them
using their own words.
185
Eye models, diagrams, and
written materials can also be used to aid in
understanding.
Shared decision-making increases patient/parent/
caregiver satisfaction with the examination and
consultation, and may improve health outcomes. The
available options, with their benefits and risks, need to
be described and patient/parent/caregiver views and
preferences elicited, before agreeing on a course of
action.
186
Language and cultural differences or misunderstandings
may prevent some individuals from accepting a doctor’s
recommendation. When communicating with patients/
parents/caregivers, it is important to take their level of

30
“health literacy” into consideration.
187
Health literacy is
“the degree to which individuals have the capacity to
obtain, process, and understand basic health information
and services needed to make appropriate decisions
regarding their health.”
188
Limited health literacy has been
associated with a range of adverse health outcomes
including decreased use of preventive services and poor
disease specific outcomes.
189
In addition, anxiety reduces the effectiveness of
patient-practitioner communications and results in
reduced attention, recall of information, and compliance
with treatment. The use of “patient-centered”
communications and “active listening” can help
reduce anxiety and improve patient/parent/caregiver
satisfaction and outcomes.
190
Improved doctor-patient
communications and higher levels of patient/parent/
caregiver involvement in care are linked to better clinical
outcomes.
191
In compliance with the Americans with Disabilities Act
(ADA), reasonable accommodations need to be made
to ensure that whatever is written or spoken is clear
and understandable to individuals with disabilities.
Appropriate auxiliary aids and services must be
made available, when needed, to enable effective
communications when evaluating, treating, or counseling
persons with hearing, vision, or speech impairments.
According to the ADA, auxiliary aids and services for
individuals who are hearing impaired include qualified
interpreters, note takers, computer-aided transcription
services, written materials, telephone handset amplifiers,
assistive listening systems, telephones compatible with
hearing aids, closed caption decoders, open and closed
captioning, telecommunications devices for the deaf
(TDD’s), videotext displays and exchange of written
notes. For individuals with vision impairments, auxiliary
aids and services include qualified readers, taped texts,
audio recordings, magnification software, optical readers,
Braille materials, and large print materials. Examples
for individuals with speech impairments include
TDD’s, computer terminals, speech synthesizers, and
communication boards.
192
Language interpreters may also be needed to assist
patients who have limited English proficiency. Family
members of patients may act as interpreters, with the
parent/caregiver consent for minors.
CONSENSUS-BASED ACTION STATEMENT:
At the conclusion of a comprehensive pediatric
eye and vision examination, the diagnosis should
be explained to the patient/parent/caregiver and
related to the patient’s symptoms, and a treatment
plan and prognosis discussed.
Evidence Quality: There is a lack of published
research to support or refute the use of this
recommendation
Benefit and Harm Assessment: Implementing
this recommendation is likely to increase patient/
parent/caregiver understanding of any diagnosed
eye or vision problems and improve compliance
with any recommended treatment. The benefits of
this recommendation were established by expert
consensus opinion
Patient/parent/caregiver counseling and education may
include:
• Review of the child’s visual and ocular health
status in relation to his/her visual symptoms and
complaints
• Discussion of any refractive correction that
provides improved visual efficiency and/or
appropriate eye protection
• Information on learning-related vision problems
• Explanation of available treatment options for
diagnosed eye or vision conditions, including
risks, benefits, and expected outcomes
• Recommendation of a course of treatment with
the reasons for its selection and the prognosis
• Discussion of the importance of patient
compliance with the treatment prescribed
• Recommendation for follow-up care, re-
examination, or referral.
When appropriate, patients/parents/caregivers should
also be counseled about:
31
a. Eye Safety and Protection
Eye injury is a leading cause of monocular blindness in
the United States and a common reason for eye-related
emergency department visits. Eye injuries treated in U.S.
hospital emergency rooms among children younger than
18 years of age averaged over 70,000 annually in 1990
through 2009.
193
(See Table 6.) The risk for eye injuries in
children is highest among 15 to 17 year olds. The most
common eye injuries are due to abrasions or foreign
bodies.
194
The majority of eye injuries in children occur at home.
193
Frequent causes are sports and recreation activities,
chemicals, or household products.
193,195
Most eye injuries
are preventable with appropriate use of protective
eyewear;
196, 197
however, in a National Health Interview
Survey of children participating in activities that can
cause eye injury, only 14.5% were reported to wear
protective eyewear all or most of the time. Older children
(12 to 17 years of age) were more likely to use protective
eyewear than younger children.
198
Table 6: Most Common Pediatric Eye Injuries
Treated in U.S. Emergency Departments
Common Pediatric Eye Injuries
Sports and recreation (e.g., basketball, baseball, football, playground
equipment)
Household chemicals (e.g., cleaning agents, bleach, pesticides)
Housewares and furniture (e.g., microwaves, flatware, tables)
Toys
Desk supplies (e.g., pens, pencils, scissors)
Tools and hardware (e.g., hammers, nails)
BB and pellet guns
Tobacco products (e.g., cigarettes, cigars, pipes)
Fireworks
Source: Rankings of common pediatric eye injuries
as reported in Pediatric eye injuries treated in U.S.
emergency departments, 1990-2009.
193
It is important to discuss eye safety issues with children/
parents/caregivers, including eye hazards at school
or home, and during sports and recreational activities,
and to promote the use of appropriate protective
eyewear to help reduce the incidence of eye injuries
among children.
193
(Evidence Grade: B),
199
(Evidence
Grade: B) Prevention strategies should focus on the
use of protective eyewear, parental supervision, and on
childhood education about both the risks of eye injury
and the utility of protective eyewear.
194
(Evidence Grade:
B)
200
EVIDENCE-BASED ACTION STATEMENT:
Parents/caregivers and children should be
educated about potential risks for eye injuries
at home, at school, and during sports and
recreational activities, and advised about safety
precautions to decrease the risk of ocular
injury.
193,199
Prevention of eye injuries in children
should focus on the use of protective eyewear,
parental supervision, and include education about
both the risks of eye injury and the benefits of
protective eyewear.
194
Evidence Quality: Grade B: Retrospective cohort
studies
Level of Confidence: Medium
Clinical Recommendation Strength: Strong
Recommendation. This recommendation should
be followed unless clear and compelling rationale
for an alternative approach is present.
Evidence Statements: It is important to
discuss eye safety issues with children/parents/
caregivers.
193
(Evidence Grade: B),
199
(Evidence
Grade: B)
Prevention strategies should focus on the use
of protective eyewear, parental supervision, and
on childhood education about both the risks of
eye injury and the utility of protective eyewear.
194
(Evidence Grade: B)
Potential Benefits:
Reduction in eye
injuries in children
Potential Risks/
Harms: None
Benefit and Harm Assessment: Benefits
significantly outweigh harms
Potential Costs: Direct cost of counseling as part
of a pediatric eye and vision examination

32
Value Judgments: None
Role of Patient Preferences: Large
Intentional Vagueness: Specific type/form of
counseling is not stated, as it is patient specific
Gaps in Evidence: Research is needed to
determine the risks and methods of eye protection
associated with specific eye injuries in children in
order to design appropriate prevention strategies
b. Ultraviolet Radiation and Blue Light Protection
Children/parents/caregivers should be advised about the
need to protect children’s eyes from excessive exposure
to sunlight. Sunlight is comprised of ultraviolet (UVA
and UVB) radiation and short wavelength visible energy
(blue light), which can cause acute effects in the eye
and may also lead to chronic effects over the life of the
individual. The eyes of infants and young children are
known to have a higher level of UV and short wavelength
transmittance than older children and adults, making
them more susceptible to energy-related injury.
201, 202
Exposure to high levels of UV-containing sunlight,
especially when reflected from snow, can cause
acute photokeratitis and keratoconjunctivitis. Chronic
exposure to even low levels of UV radiation is a risk
factor for developing cataracts, pterygium, squamous
cell carcinoma of the cornea and conjunctiva, and skin
cancer.
203
Epidemiological evidence also shows that
excess chronic sunlight exposure leads to a significantly
increased risk for developing age-related macular
degeneration as an older adult.
204
Exposure to high levels of short wavelength visible
energy (blue light) also has the potential to cause
photochemical retinal damage, which is known to occur
with direct sun viewing.
205, 206
In addition, the increased
evening use of laptops and other broad spectrum self-
illuminated devices rich in blue light has been suggested
to interfere with good sleep hygiene, especially in
adolescents.
207
Children can reduce the potential for eye damage from
UV radiation and blue light by not looking directly at the
sun, and wearing sunglasses and/or clear prescription
lenses and brimmed hats when outdoors.
CONSENSUS-BASED ACTION STATEMENT:
All children and their parents/caregivers should
be advised about the benefits of the regular use
of sunglasses and/or clear prescription glasses
that effectively block at least 99% of UVA and
UVB radiation, the use of hats with brims when
outdoors, and the importance of not looking
directly at the sun.
Evidence Quality: There is a lack of published
research to support or refute the use of this
recommendation.
Benefit and Harm Assessment: Implementing
this recommendation is likely to decrease patient
risk of eye health problems from acute or chronic
exposure to UV radiation and blue light. The
benefits of this recommendation were established
by expert consensus opinion.
c. Impact of Near Work and Reduced Time Outdoors
on Vision
The prevalence of myopia in children has been increasing
significantly in the past few decades.
44
Environmental
factors such as time spent on reading and other near
activities and the limited amount of time spent outdoors
have been cited as potential factors contributing to
the development and progression of myopia.
208
Most
children spend considerable time each day using
computers, tablets, or smart phones at school and
at home. As a result, they may be spending less time
outdoors.
Although there is conflicting evidence, more time spent
outdoors and less time indoors doing near work may
slow myopia progression and prevent high myopia.
208
(Evidence Grade: A),
209
(Evidence Grade: B),
210
(Evidence
Grade: B),
211
(Evidence Grade: D).
212
EVIDENCE-BASED ACTION STATEMENT:
Patients/parents/caregivers should be counseled
about the benefits to children’s vision of spending
more time outdoors.
208-211

33
Evidence Quality: Grade B. Randomized clinical
trial, Prospective cohort studies, Cross-sectional
study
Level of Confidence: Medium
Clinical Recommendation Strength:
Recommendation. This recommendation should
generally be followed, but remain alert for new
information.
Evidence Statements: More time spent outdoors
and less time indoors doing near work may slow
axial elongation and prevent high myopia thereby
reducing the risk of developing sight-threatening
conditions such as retinal detachment and myopic
retinopathy.
208
(Evidence Grade: A)
More time outside may decrease myopia
progression. Less outdoor/sports activity before
myopia onset may exert a stronger influence on
the development of myopia than near work.
209
(Evidence Grade: B)
Outdoor time and near work do not have a major
effect on myopia progression.
210
(Evidence Grade:
B)
Higher levels of outdoor activity were associated
with lower amounts of myopia in primary school
students.
211
(Evidence Grade: D)
Potential Benefits:
Implementation of this
recommendation is
likely to help reduce
the development and
progression of myopia
in children
Potential Risks/
Harms: None
Benefit and Harm Assessment: Benefits
significantly outweigh harms
Potential Costs: Direct cost of counseling as
part of a pediatric eye and vision examination and
parental/caregiver time off from work
Value Judgments: None
Role of Patient Preferences: Moderate
Intentional Vagueness: Specific type/form of
counseling is not stated, as it is patient specific
Gaps in Evidence: Research is needed on the
effects and possible interaction of outdoor activity
and near work on myopia in children
d. Myopia Control
Childhood is the preferred time to consider the use of
myopia control procedures, as early onset myopia is
associated with higher progression rates and increased
risk of continuing to high myopia.
213
The use of progressive addition spectacle lenses,
prismatic bifocals, multiple and dual focus contact
lenses, orthokeratology, and atropine have been studied
to slow myopia progression.
214
The approaches to
control of myopia that have been shown in studies to
be most successful are the use of low concentrations of
atropine eye drops
215
and orthokeratology.
214, 216, 217
.
Parents/caregivers of children who are at risk for
developing or have developed myopia should be
counseled about the potential complications of myopia
progression and the treatment options available for its
control.
4. Coordination and Frequency of Care
The diagnosis of a wide array of eye and vision
anomalies, diseases, disorders, and related systemic
conditions may result from a comprehensive pediatric
eye and vision examination. The nature and severity of
the problem(s) diagnosed determine the need for:
• Optical correction
• Vision therapy
• Vision rehabilitation services
• Prescription or nonprescription medications
• Surgery
• Follow-up for additional evaluation and/or
treatment.
a. Coordination of Care
Based on the examination, it may be determined that the
patient needs additional services. These may include:

34
• Intraprofessional consultation with another
doctor of optometry for treatment and
management of ocular disease, vision
rehabilitation, vision therapy, and/or specialty
contact lenses.
• Interprofessional consultation with an
ophthalmologist may be necessary for
ophthalmic surgery or other aspects of
secondary or tertiary eye care.
• Some vision problems can interfere with
learning. Children at risk for learning-related
vision problems should be evaluated by a doctor
of optometry.
• Referral for consultation with the child’s
pediatrician or other primary care physician,
developmental pediatrician, pediatric neurologist,
the school system, a child psychologist or
psychiatrist, or the local or state Department of
Special Education should be considered when
problems in other developmental areas such as
behavior, language, or social development are
suspected or when a full psychoeducational
evaluation is indicated.
• The comprehensive pediatric eye and vision
examination may reveal non-ophthalmic
conditions for which coordination of care may
be needed. The patient may be referred to
his or her pediatrician/primary care physician
or another health care provider for further
evaluation and treatment of systemic conditions
or related health problems. Information shared
with other health care providers offers a unique
and important perspective resulting in an
improved team approach to interdisciplinary care
of the patient.
• Ocular telehealth programs may be a
component of care for some patients,
particularly in areas where access to specialized
eye care services is limited. The use of ocular
telehealth-based programs has the potential
to expand access to eye care services;
however, telehealth-based evaluations are not
a substitute for an in-person comprehensive
eye examination. These programs rely on the
digital capture and transmission of standardized
ocular images and patient health information at
one location for interpretation and evaluation at
another location by trained observers who can
recommend a treatment and care plan. To date,
telehealth programs have been most widely
used for the evaluation of patients with diabetic
retinopathy.
218
b. Frequency of Care
Children should receive periodic eye and vision
examinations to diagnose and treat any eye disease in
its early stages in order to prevent or minimize vision
loss and maximize visual abilities. These examinations
can also identify problems that may be affecting visual
function and achievement at school, at home, and in
sports or leisure activities. In addition, the early signs
and symptoms of systemic medical conditions, such
as diabetes, may be revealed during a comprehensive
pediatric eye and vision examination.
The recommended frequency of a comprehensive
pediatric eye and vision examination (Table 7) varies
with a child’s age, ocular and medical history, and other
related risk factors.
• Infants and Toddlers (newborn through 2 years
of age)
Clinical experience and research have shown that at
6 months the average child has reached a number
of critical developmental milestones, making this an
appropriate age for the first eye and vision examination.
Within the first 6 months of life, rapid changes occur
in most components of the visual system including
visual acuity,
121, 219
accommodation,
220, 221
and binocular
vision.
222-224
Since the developing visual system is
considered most susceptible to interference during the
first few years of life,
225-227
interference during this critical
phase of development may have significant long-term
effects; therefore, early diagnosis and treatment are
critical to avoid vision loss.
There is a high prevalence of eye and vision problems in
preterm children.
228
Preterm infants with a history of ROP
should be closely monitored for the development of high

35
myopia, astigmatism, anisometropia,
229
(Evidence Grade:
B) strabismus,
76
and other ocular diseases.
One of the primary goals of examining young children is
to detect amblyopia so that treatment can be initiated
as early as possible. Early visual examination of infants
for amblyopia and amblyopic risk factors can lower
the prevalence and severity of amblyopia in children.
230
(Evidence Grade: B)
Assessment of infant refractive error can identify not
only vision problems, but also potential developmental
difficulties. Infants with hyperopia may show deficits
in many visuocognitive, spatial, visuomotor, and
attention tests.
231
(Evidence Grade: B) Significant
hyperopia is commonly found in association with the
early development of strabismus and amblyopia, with
increased risk of development by age 4 years.
The wearing of a partial correction for significant
hyperopia and anisometropia throughout infancy can
reduce the incidence of poorer than average visual acuity
in 3 to 5 1/2 year olds.
232
Spectacle correction in infancy
also improves the chances of infants with hyperopia
having normal vision at age 4 and beyond.
233
EVIDENCE-BASED ACTION STATEMENT:
Infants should receive an in-person comprehensive
eye and vision assessment between 6 and 12
months of age for the prevention and/or early
diagnosis and treatment of sight-threatening eye
conditions and to evaluate visual
development.
229-231
Evidence Quality: Grade B: Prospective cohort
studies, Diagnostic study
Level of Confidence: High
Clinical Recommendation Strength: Strong
Recommendation. This recommendation should
be followed unless clear and compelling rationale
for an alternative approach is present.
Evidence Statements: Preterm infants with a
history of ROP should be closely monitored for the
development of high myopia, astigmatism, and
anisometropia.
229
(Evidence Grade: B)
Early visual examination in infants for amblyopia
and amblyopic risk factors can lower the
prevalence and severity of amblyopia in children.
230
(Evidence Grade: B)
Assessment of infant refractive error can identify
not only vision problems, but also potential
developmental difficulties. Hyperopic infants may
show deficits in many visuocognitive, spatial,
visuomotor, and attention tests.
231
(Evidence
Grade: B)
Potential Benefits:
Early identification and
treatment of eye and
vision problems
Potential Risks/
Harms: None
Benefit and Harm Assessment: Benefits
significantly outweigh harms
Potential Costs: Direct cost of testing and
parent/caregiver time off from work
Value Judgments: None
Role of Patient Preferences: Moderate
Intentional Vagueness: None
Gaps in Evidence: None identified
• Preschool Children (3 through 5 years of age)
Vision care in preschool children is very important
because their visual system is still developing. They are
at risk for the development of amblyopia, strabismus,
and refractive error, which may lead to long term visual
impairment.
40, 41, 53, 234-236
Amblyopia is a treatable condition in children and
adolescents
54
(Evidence Grade: A); however, amblyopia
is more responsive to treatment among children younger
than 7 years of age.
54-60
Significant uncorrected refractive
errors are a risk factor for the development of amblyopia.
In addition to its impact on vision, amblyopia can affect
an individual’s psychosocial functioning, warranting early
diagnosis and treatment.
19

36
Uncorrected refractive errors have been associated with
delays in development of cognitive ability and motor
skill.
10, 231, 237
The Vision in Preschoolers-Hyperopia in
Preschoolers (VIP-HIP) study found that uncorrected
hyperopia ≥4.00D, as well as uncorrected hyperopia
≥3.00D to ≤6.00D in conjunction with reduced
binocular visual acuity (20/40 or worse) or reduced
near stereoacuity (240 seconds of arc or worse), are
associated with significantly worse performance on
a test of preschool early literacy (TOPEL) in 4 and
5 year old children.
238
(Evidence Grade: C) Children
with astigmatism tend to score lower on measures of
academic and developmental skills than children without
astigmatism.
239
Spectacle correction of children with
astigmatism during the preschool years can also result in
significantly improved best-corrected visual acuity by the
time they reach kindergarten age.
240
(Evidence Grade: C)
Uncorrected vision problems can have a detrimental
effect on vision development, learning, school success,
and socialization. Many eye and vision problems are
asymptomatic in this age range; therefore, it is important
that preschool children receive a comprehensive eye
examination. While the U.S. Preventive Services Task
Force recommends that children have their vision
screened at least once between the ages of 3 and 5
years,
107
(Evidence Grade: B) gaps exist in the delivery
of preschool vision screening. Rates of vision screening
in preschool children are low, particularly in 3 year old
children.
241
(Evidence Grade: C)
EVIDENCE-BASED ACTION STATEMENT:
Preschool age children should receive an in-person
comprehensive eye and vision examination at least
once between the ages of 3 and 5 to prevent and/
or diagnose and treat any eye or vision conditions
that may affect visual development.
54,107, 238, 240, 241
Evidence Quality: Grade B. Systematic Review,
Case series, Cross-sectional study
Level of Confidence: Medium
Clinical Recommendation Strength: Strong
Recommendation. This recommendation should
be followed, unless clear and compelling rationale
for an alternative approach is present.
Evidence Statements: Amblyopia is a treatable
condition in children and adolescents
54
(Evidence
Grade: A); however amblyopia is more responsive
to treatment among children younger than 7 years
of age.
Uncorrected hyperopia in 4 and 5 year old
children has been associated with delays in the
development of early literacy.
238
(Evidence Grade:
C)
Spectacle correction of astigmatism during the
preschool years can result in significantly improved
best-corrected visual acuity by kindergarten age.
240
(Evidence Grade: C)
The U.S. Preventive Services Task Force
recommends that children have their vision
screened at least once between the ages of
3 and 5 years of age;
107
(Evidence Grade: B)
however, gaps exist in the delivery of preschool
vision screening and rates of screening are low,
particularly in 3 year old children.
241
(Evidence
Grade: C)
Potential Benefits:
Early identification and
treatment of eye and
vision problems
Potential Risks/
Harms: None
Benefit and Harm Assessment: Benefits
significantly outweigh harms
Potential Costs: Direct cost of testing and
parent/caregiver time off from work
Value Judgments: None
Role of Patient Preferences: Moderate
Intentional Vagueness: None
Gaps in Evidence: None identified
• School-age Children (6 through 11 and 12
through 18 years of age)
Vision may change frequently during the school years.
The most common problems are due to the
development and progression of refractive errors.
Myopia generally occurs in children during their early
school years and increases in magnitude as they get

37
older. If myopia is defined as 0.50D or more, the
percentage of children becoming myopic is estimated to
be 23.4%. The age at onset ranges from 7 to 16 years.
Sixteen percent of children enrolled in the CLEERE study
developed myopia (0.75D or more) during their school-
age years. The highest percentage of new cases
occurred at age 11.
42
Children should receive an eye examination at the
beginning of primary school to test for the presence
of myopia
115
(Evidence Grade: B) and, if diagnosed,
they should have a comprehensive examination at least
annually or as frequently as their doctor recommends
because of rapid myopia progression.
242
(Evidence
Grade: B) Children with myopia, especially those younger
than 9 years of age and/or with two parents with myopia,
are at higher risk for myopia progression and should be
examined more than once per year.
208
(Evidence Grade:
A)
In addition to its relationship to the development of
strabismus and amblyopia, hyperopia can also affect the
development of literacy skills. Children with uncorrected
hyperopia show reduced performance in the acquisition
of emergent literacy skills.
238
(Evidence Grade: C),
243
(Evidence Grade: C) Correction of hyperopia may, under
specific conditions, lead to increased reading speed;
therefore, eye examinations to diagnose uncorrected
hyperopia are recommended.
244
(Evidence Grade: B)
An accommodative or vergence dysfunction can have
a negative effect on a child’s school performance,
especially after third grade when the child must read
smaller print and reading demands increase. Children
with convergence insufficiency self-report more problems
compared to children with normal binocular vision.
245
These include somatic (e.g., eyes hurt or headaches),
visual (e.g., blur and diplopia), and performance (e.g.,
loss of concentration, frequent need to re-read and
difficulty remembering what is read) problems. Due to the
discomfort of these symptoms, a child may not be able
to complete reading or homework assignments and may
be easily distracted or inattentive.
Studies have reported an association between reading
and eye movements.
246-248
Efficient reading requires
accurate eye movements. Treatment of children with eye
movement problems has been shown to improve reading
comprehension.
248
Diagnosis and treatment of an accommodative or
vergence problem may reduce the negative impact on
academic performance.
65
(Evidence Grade B)
249
Vision
therapy has been shown to be effective in improving
accommodative amplitude and accommodative facility
in school-age children with symptomatic convergence
insufficiency and accommodative dysfunction.
Children with Attention Deficit/Hyperactivity Disorder
(AD/HD) have been reported to have a much greater
incidence of CI than those without AD/HD;
250
therefore,
these children may benefit from comprehensive vision
evaluation to assess the presence of convergence
insufficiency.
251
(Evidence Grade: D) Treatment of
convergence insufficiency has been associated with
reduction in the frequency of adverse academic
behaviors.
65
(Evidence Grade B)
67
Click to view the (AOA Clinical Practice Guidelines
web page)
EVIDENCE-BASED ACTION STATEMENT:
School-age children should receive an in-person
comprehensive eye and vision examination before
beginning school to diagnose, treat, and manage
any eye or vision conditions.
65, 115, 238, 243, 244, 251
Evidence Quality: Grade B. Prospective cohort
studies, Case-control study, Cross-sectional study.
Level of Confidence: Medium
Clinical Recommendation Strength: Strong
Recommendation. This recommendation should
be followed unless clear and compelling rationale
for an alternative approach is present.

38
Evidence Statements: Children should receive
an eye examination at the beginning of primary
school to diagnose the onset of myopia.
115
(Evidence Grade: B)
Hyperopia can affect the development of literacy
skills. Children with uncorrected hyperopia
show reduced performance in the acquisition of
emergent literacy skills.
238
(Evidence Grade: C),
243
(Evidence Grade: C)
Correction of hyperopia may, under specific
conditions, lead to increased reading speed;
therefore, eye examinations to diagnose
uncorrected hyperopia are recommended.
244
(Evidence Grade: B)
Early diagnosis and treatment of an
accommodative or vergence problem may reduce
the negative impact on academic performance.
65
(Evidence Grade: B)
Children with AD/HD or related learning problems
may benefit from comprehensive vision evaluation
to assess the presence of convergence
insufficiency.
251
(Evidence Grade: D)
Treatment of convergence insufficiency has been
associated with reduction in the frequency of
adverse academic behaviors.
65
(Evidence Grade B)
Potential Benefits:
Early identification and
treatment of eye and
vision problems
Potential Risks/
Harms: None
Benefit and Harm Assessment: Benefits
significantly outweigh harms
Potential Costs: Direct cost of testing and
parent/caregiver time off from work
Value Judgments: None
Role of Patient Preferences: Moderate
Intentional Vagueness: None
Gaps in Evidence: None identified
EVIDENCE-BASED ACTION STATEMENT:
Children with myopia should have an in-person
comprehensive eye and vision examination at
least annually, or as frequently as recommended
(especially until age 12), because of the potential
for rapid myopia progression.
208, 242
Evidence Quality: Grade B. Randomized clinical
trial, Prospective cohort study
Level of Confidence: Medium
Clinical Recommendation Strength: Strong
Recommendation. This recommendation should
be followed unless clear and compelling rationale
for an alternative approach is present.
Evidence Statements: Children with myopia
should have an examination at least annually or as
frequently as their doctor recommends until the
age of 12 because of rapid myopia progression.
242
(Evidence Grade: B)
When both parents have myopia, children are
at higher risk for progression and should be
examined more than once per year.
208
(Evidence
Grade: A)
Potential Benefits:
Early identification and
treatment of eye and
vision problems
Potential Risks/
Harms: None
Benefit and Harm Assessment: Benefits
significantly outweigh harms
Potential Costs: Direct cost of testing and
parent/caregiver time off from work
Value Judgments: None
Role of Patient Preferences: Moderate
Intentional Vagueness: None
Gaps in Evidence: None identified
CONSENSUS-BASED ACTION STATEMENT:
School-age children should receive an in-person
comprehensive eye and vision examination
annually to diagnose, treat, and manage any eye
or vision problems.

39
Evidence Quality: There is a lack of published
research to support or refute the use of this
recommendation.
Benefit and Harm Assessment: Implementing
this recommendation is likely to result in earlier
diagnosis and treatment of eye and vision
problems and improved visual function. The
benefits of this recommendation were established
by expert consensus opinion.
c. At-risk Children
The extent to which a child is at risk for the development
of eye and vision problems determines the appropriate
re-evaluation schedule. Children with ocular signs and
symptoms require a prompt comprehensive examination.
Furthermore, the presence of certain risk factors may
necessitate more frequent examinations based on
professional judgment. Factors placing an infant, toddler,
or child at significant risk for eye and vision problems
include:
• Prematurity, low birth weight, prolonged
supplemental oxygen at birth
• Family history of myopia, amblyopia, strabismus,
retinoblastoma, congenital cataracts, metabolic,
or genetic disease
• Infection of mother during pregnancy (e.g.,
rubella, toxoplasmosis, venereal disease,
herpes, cytomegalovirus, or human
immunodeficiency virus)
• Maternal smoking, use of alcohol, or illicit drug
use during pregnancy
• Cortical visual impairment
• Difficult or assisted labor, which may be
associated with fetal distress
• High or progressive refractive error
• Strabismus
• Anisometropia
• Academic performance problems
• Known or suspected neurodevelopmental
disorders
• Systemic health conditions with potential ocular
manifestations
• Wearing contact lenses
• Having functional vision in only one eye
• Eye surgery or previous eye injury
• Taking prescription or nonprescription
drugs (e.g., over the counter medications,
supplements, herbal remedies) with potential
ocular side effects
Table 7: Recommended Eye Examination
Frequency for the Pediatric Patient**
Examination Interval
Patient Age
Asymptomatic/
Low Risk
At-risk
Birth through 2 years
At 6 to12 months
of age
At 6 to 12 months
of age or as
recommended
3 through 5 years
At least once between
3 and 5 years of age
At least once between
3 and 5 years of age
or as recommended
6 through 18 years
Before first grade and
annually thereafter
Before first grade
and annually, or
as recommended,
thereafter
**The American Optometric Association Clinical
Practice Guidelines provide more information on
other eye and vision disorders and their risk factors.
Click to view the (AOA Clinical Practice Guidelines
web page)
D. Conclusion
The prevalence of eye and vision disorders is substantial
in children. Research indicates that early detection and
intervention are particularly important in children because
of the rapid development of the visual system in early
childhood and its sensitivity to interference. When visual
disorders such as amblyopia, strabismus, non-strabismic
binocular vision disorders, and significant refractive
error are undetected, the long-term consequences can

40
lead to significant vision loss, decreased educational
and occupational opportunities, and reduced quality
of life. In addition, the cost of providing appropriate
treatment for longstanding eye and vision disorders may
be significantly higher than the cost of diagnosing and
treating these problems early in life. A comprehensive
pediatric eye and vision examination by a doctor of
optometry is imperative for the timely diagnosis and
treatment of eye and vision problems.

41
IV. REFERENCES
1. Institute of Medicine Committee on Standards for Developing Trustworthy Clinical Practice Guidelines. Clinical Practice
Guidelines We Can Trust. The National Academies Press. 2011:Washington, D.C.
2. Multi-Ethnic Pediatric Eye Disease Study Group. Prevalence of myopia and hyperopia in 6- to 72-month-old African
American and Hispanic children: the Multi-Ethnic Pediatric Eye Disease Study. Ophthalmology 2010; 117:140-47.
3. Wen G, Tarczy-Hornoch K, McKean-Cowdin R, et al. Prevalence of myopia, hyperopia, and astigmatism in non-Hispanic
white and Asian children: Multi-Ethnic Pediatric Eye Disease Study. Ophthalmology 2013; 120:2109-16.
4. Fozailoff A, Tarczy-Hornoch K, Cotter S, et al. Prevalence of astigmatism in 6- to 72-month-old African American and
Hispanic children: the Multi-Ethnic Pediatric Eye Disease Study. Ophthalmology 2011; 118:284-93.
5. Giordano L, Friedman DS, Repka MX, et al. Prevalence of refractive error among preschool children in an urban population:
the Baltimore Pediatric Eye Disease Study. Ophthalmology 2009; 116:739-46.
6. Multi-Ethnic Pediatric Eye Disease Study Group. Prevalence of amblyopia and strabismus in African American and Hispanic
children ages 6 to 72 months: the Multi-Ethnic Pediatric Eye Disease Study. Ophthalmology 2008; 115:1229-36.
7. Friedman DS, Repka MX, Katz J, et al. Prevalence of amblyopia and strabismus in white and African American children
aged 6 through 71 months: the Baltimore Pediatric Eye Disease Study. Ophthalmology 2009; 116:2128-34.
8. McKean-Cowdin R, Cotter SA, Tarczy-Hornoch K, et al. Prevalence of amblyopia or strabismus in Asian and non-Hispanic
white preschool children: Multi-Ethnic Pediatric Eye Disease Study. Ophthalmology 2013; 120:2117-24.
9. Kemper AR, Bruckman D, Freed GL. Prevalence and distribution of corrective lenses among school-age children. Optom
Vis Sci 2004; 81:7-10.
10. Roch-Levecq AC, Brody BL, Thomas RG, Brown SI. Ametropia, preschoolers’ cognitive abilities, and effects of spectacle
correction. Arch Ophthalmol 2008; 126:252-58.
11. Atkinson J, Nardini M, Anker S, et al. Refractive errors in infancy predict reduced performance on the movement
assessment battery for children at 3 1/2 and 5 1/2 years. Dev Med Child Neurol 2005; 47:243-51.
12. Kulp MT, Schmidt PP. Visual predictors of reading performance in kindergarten and first grade children. Optom Vis Sci
1996; 73:255-62.
13. Simons HD, Grisham JD. Binocular anomalies and reading problems. J Am Optom Assoc 1987; 58:578-87.
14. Maples WC. Visual factors that significantly impact academic performance. Optometry 2003; 74:35-49.
15. Goldstand S, Koslowe KC, Parush S. Vision, visual-information processing, and academic performance among seventh-
grade schoolchildren: a more significant relationship than we thought? Am J Occup Ther 2005; 59:377-89.
16. Basch CE. Vision and the achievement gap among urban minority youth. J Sch Health 2011; 81:599-605.
17. Mojon-Azzi SM, Kunz A, Mojon DS. Strabismus and discrimination in children: are children with strabismus invited to
fewer birthday parties? Br J Ophthalmol 2011; 95:473-76.
18. Webber AL, Wood JM, Gole GA, Brown B. Effect of amblyopia on self-esteem in children. Optom Vis Sci 2008; 85:1074-
81.

42
19. Packwood EA, Cruz OA, Rychwalski PJ, Keech RV. The psychosocial effects of amblyopia study. J AAPOS 1999; 3:15-
17.
20. Davidson S, Quinn GE. The impact of pediatric vision disorders in adulthood. Pediatrics 2011; 127:334-39.
21. Menacker SJ. Visual function in children with developmental disabilities. Pediatr Clin North Am 1993; 40:659-74.
22. Akinci A, Oner O, Bozkurt OH, et al. Refractive errors and ocular findings in children with intellectual disability: a controlled
study. J AAPOS 2008; 12:477-81.
23. Akinci A, Oner O, Bozkurt OH, et al. Refractive errors and strabismus in children with Down syndrome: a controlled study.
J Pediatr Ophthalmol Strabismus 2009; 46:83-86.
24. Black K, McCarus C, Collins ML, Jensen A. Ocular manifestations of autism in ophthalmology. Strabismus 2013; 21:98-
102.
25. Ikeda J, Davitt BV, Ultmann M, et al. Brief report: incidence of ophthalmologic disorders in children with autism. J Autism
Dev Disord 2013; 43:1447-51.
26. Woodhouse JM. Investigating and managing the child with special needs. Ophthalmic Physiol Opt 1998; 18:147-52.
27. Salt A, Sargent J. Common visual problems in children with disability. Arch Dis Child 2014; 99:1163-68.
28. Brémond-Gignac D, Copin H, Lapillonne A, et al. Visual development in infants: physiological and pathological
mechanisms. Curr Opin Ophthalmol 2011; 22:S1-S8.
29. Atkinson J. The Developing Visual Brain. Oxford: Oxford University Press; 2002.
30. Sokol, S. Measurement of infant visual acuity from pattern reversal evoked potentials. Vision Res 1978; 18:33-39.
31. Ciner EB, Schanel-Klitsch E, Herzberg C. Stereoacuity development: 6 months to 5 years. A new tool for testing and
screening. Optom Vis Sci 1996; 73:43-48.
32. Birch EE, Morale SE, Jeffrey BG, et al. Measurement of stereoacuity outcomes at ages 1 to 24 months: Randot
Stereocards. J AAPOS 2005; 9:31-36.
33. Tarczy-Hornoch K. Accommodative lag and refractive error in infants and toddlers. J AAPOS 2012; 16:112-17.
34. Scheiman M, Herzberg H, Frantz K, Margolies M. Normative study of accommodative facility in elementary schoolchildren.
Am J Optom Physiol Opt 1988; 65:127-34.
35. Fioravanti F, Inchingolo P, Pensiero S, Spanio M. Saccadic eye movement conjugation in children. Vision Res 1995;
35:3217-28.
36. Yang Q, Kapoula Z. Binocular coordination of saccades at far and at near in children and in adults. J Vis 2003; 3:554-61.
37. Scheiman M, Herzberg H, Frantz K, Margolies M. A normative study of step vergence in elementary schoolchildren. J Am
Optom Assoc 1989; 60:276-80.
38. Tarczy-Hornoch K, Cotter SA, Borchert M, et al. Prevalence and causes of visual impairment in Asian and non-Hispanic
white preschool children: Multi-Ethnic Pediatric Eye Disease Study. Ophthalmology 2013; 120:1220-26.

43
39. Multi-Ethnic Pediatric Eye Disease Study Group. Prevalence and causes of visual impairment in African-American and
Hispanic preschool children: the Multi-Ethnic Pediatric Eye Disease Study. Ophthalmology 2009; 116:1990-2000.
40. Cotter SA, Varma R, Tarczy-Hornoch K, et. al. Risk factors associated with childhood strabismus: the Multi-Ethnic
Pediatric Eye Disease and Baltimore Pediatric Eye Disease Studies. Ophthalmology 2011; 118:2251-61.
41. Pascual M, Huang J, Maguire MG, et al. Risk factors for amblyopia in the Vision in Preschoolers Study. Ophthalmology
2014; 121:622-29.
42. Kleinstein RN, Sinnott LT, Jones-Jordan LA, et al. New cases of myopia in children. Arch Ophthalmol 2012; 130:1274-79.
43. Matsumura H, Hirai H. Prevalence of myopia and refractive changes in students from 3 to 17 years of age. Surv
Ophthalmol 1999; 44 Suppl 1:S109-15.
44. Vitale S, Sperduto RD, Ferris FL 3rd. Increased prevalence of myopia in the United States between 1971-1972 and 1999-
2004. Arch Ophthalmol 2009; 127:1632-39.
45. Saw SM, Katz J, Schein OD, et al. Epidemiology of myopia. Epidemiol Rev 1996; 18:175-87.
46. Wong TY, Ferreira A, Hughes R, et al. Epidemiology and disease burden of pathologic myopia and myopic choroidal
neovascularization: an evidence-based systematic review. Am J Ophthalmol 2014; 157:9-25.
47. Gwiazda J, Mohindra I, Brill S, Held R. Infant astigmatism and meridional amblyopia. Vision Res 1985; 25:1269-76.
48: Mutti DO, Mitchell GL, Jones LA, et al. Refractive astigmatism and the toricity of ocular components in human infants.
Optom Vis Sci 2004; 81:753-61.
49. Borchert M, Tarczy-Hornoch K, Cotter SA, et al. Anisometropia in Hispanic and African American infants and young
children: the Multi-Ethnic Pediatric Eye Disease Study. Ophthalmology 2010; 117:148-53.
50. Abrahamsson M, Sjostrand J. Natural history of infantile anisometropia. Br J Ophthalmol 1996; 80:860-63.
51. Afsari S, Rose KA, Gole GA, et al. Prevalence of anisometropia and its association with refractive error and amblyopia in
preschool children. Br J Ophthalmol 2013; 97:1095-99.
52. Kleinstein RN, Jones LA, Hullett S, et al. Refractive error and ethnicity in children. Arch Ophthalmol 2003; 121:1141-47.
53. Tarczy-Hornoch K, Varma R, Cotter SA, et al. Risk factors for decreased visual acuity in preschool children: the Multi-
Ethnic Pediatric Eye Disease and Baltimore Pediatric Eye Disease Studies. Ophthalmology 2011; 118:2262-73.
54. Holmes JM, Lazar EL, Melia BM, et al. Effect of age on response to amblyopia treatment in children. Arch Ophthalmol
2011; 129:1451-57.
55. Pediatric Eye Disease Investigator Group. A prospective, pilot study of treatment of amblyopia in children 10 to <18 years
old. Am J Ophthalmol 2004; 137:581-83.
56. Scheiman MM, Hertle RW, Beck RW, et al. Randomized trial of treatment of amblyopia in children aged 7 to 17 years.
Arch Ophthalmol 2005; 123:437-47.
57. Hertle RW, Scheiman MM, Beck RW, et al. Stability of visual acuity improvement following discontinuation of amblyopia
treatment in children aged 7 to 12 years. Arch Ophthalmol 2007; 125:655-59.

44
58. Scheiman MM, Hertle RW, Kraker RT, et al. Patching vs atropine to treat amblyopia in children aged 7 to 12 years: a
randomized trial. Arch Ophthalmol 2008; 126:1634-42.
59. Hess RF, Thompson B. New insights into amblyopia: binocular therapy and noninvasive brain stimulation. J AAPOS 2013;
17:89-93.
60. Hess RF, Mansouri B, Thompson B. A binocular approach to treating amblyopia: antisuppression therapy. Optom Vis Sci
2010; 87:697-704.
61. Powell C, Hatt SR. Vision screening for amblyopia in childhood. Cochrane Database Syst Rev 2009:CD005020.
62. Donnelly UM, Stewart NM, Hollinger M. Prevalence and outcomes of childhood visual disorders. Ophthalmic Epidemiol
2005; 12:243-50.
63. Scheiman M, Gallaway M, Coulter R, et al. Prevalence of vision and ocular disease conditions in a clinical pediatric
population. J Am Optom Assoc 1996; 67:193-202.
64. Cooper J, Jamal N. Convergence insufficiency-a major review. Optometry 2012; 83:137-58.
65. Borsting E, Mitchell GL, Kulp MT, et al. Improvement in academic behaviors after successful treatment of convergence
insufficiency. Optom Vis Sci 2012; 89:12-18.
66. Letourneau JE, Ducic S. Prevalence of convergence insufficiency among elementary school children. Can J Optom 1988;
50:194-97.
67. Borsting E, Mitchell GL, Arnold LE, et al. Behavioral and emotional problems associated with convergence insufficiency in
children: an open trial. J Atten Disord 2016; 20:836-44.
68. Rouse MW, Borsting E, Hyman L, et al. Frequency of convergence insufficiency among fifth and sixth graders. The
Convergence Insufficiency and Reading Study (CIRS) group. Optom Vis Sci 1999; 76:643-49.
69. Xie JZ, Tarczy-Hornoch K, Lin J, et al. Color vision deficiency in preschool children: the Multi-Ethnic Pediatric Eye Disease
Study. Ophthalmology 2014; 121:1469-74.
70. Thadani SM, Foster CS. Treatment of ocular inflammation in children. Paediatr Drugs 2004; 6:289-301.
71. Reiff A. Ocular complications of childhood rheumatic diseases: nonuveitic inflammatory eye diseases. Curr Rheumatol
Rep 2009; 11:226-32.
72. Towner SR, Michet CJ Jr., O’Fallon WM Nelson AM. The epidemiology of juvenile arthritis in Rochester, Minnesota 1960-
1979. Arthritis Rheum 1983; 26:1208-13.
73. VanderVeen DK, Bremer DL, Fellows RR, et al. Prevalence and course of strabismus through age 6 years in participants
of the Early Treatment for Retinopathy of Prematurity randomized trial. J AAPOS 2011; 15:536-40.
74. Goktas A, Sener EC, Sanac AS. An assessment of ocular morbidities of children born prematurely in early childhood. J
Pediatr Ophthalmol Strabismus 2012; 49:236-41.
75. Saldir M, Sarici SU, Mutlu FM, et al. An analysis of neonatal risk factors associated with the development of
ophthalmologic problems at infancy and early childhood: a study of premature infants born at or before 32 weeks of
gestation. J Pediatr Ophthalmol Strabismus 2010; 47:331-37.

45
76. VanderVeen DK, Allred EN, Wallace DK, Leviton A. Strabismus at age 2 years in children born before 28 weeeks
gestation: antecedents and correlates. J Child Neurol 2016; 31:451-60.
77. Faia LJ, Trese MT. Retinopathy of prematurity care: screening to vitrectomy. Int Ophthalmol Clin 2011; 51:1-16.
78. Good WV, Hardy RJ, Dobson V, et al. The incidence and course of retinopathy of prematurity: findings from the Early
Treatment for Retinopathy of Prematurity Study. Pediatrics 2005; 116:15-23.
79. Gunn DJ, Cartwright DW, Gole GA. Incidence of retinopathy of prematurity in extremely premature infants over an 18-year
period. Clin Exp Ophthalmol 2012; 40:93-99.
80. Holmes JM, Leske DA, Burke JP, Hodge DO. Birth prevalence of visually significant infantile cataract in a defined U.S.
population. Ophthalmic Epidemiol 2003; 10:67-74.
81. Aponte EP, Diehl N, Mohney BG. Incidence and clinical characteristics of childhood glaucoma: a population-based study.
Arch Ophthalmol 2010; 128:478-82.
82. Ferrari S, Di Iorio E, Barbaro V, et al. Retinitis pigmentosa: genes and disease mechanisms. Curr Genomics 2011; 12:238-
49.
83. Genetics Home Reference. http://ghr.nlm.nih.gov/condition/retinitis-pigmentosa. Accesed 6/25/2015.
84. Wong JR, Tucker MA, Kleinerman RA, Devesa SS. Retinoblastoma incidence patterns in the US Surveillance,
Epidemiology, and End Results program. JAMA Ophthalmol 2014; 132:478-83.
85. The Eye Cancer Foundation. Eye Cancer Network. http://www.eyecancer.com/conditions/42/retinoblastoma. Accessed
6/25/2015.
86. Truong B, Green AL, Friedrich P, et al. Ethnic, racial, and socioeconomic disparities in retinoblastoma. JAMA Pediatr 2015;
169:1096-104.
87. Delhiwala KS, Vadakkal IP, Mulay K, et al. Retinoblastoma: an update. Semin Diagn Pathol 2016; 33:133-40.
88. Forlenza GP, Stewart MW. Diabetic retinopathy in children. Pediatr Endocrinol Rev 2012; 10:217-26.
89. Lueder GT, Silverstein J. Screening for retinopathy in the pediatric patient with type 1 diabetes mellitus. Pediatrics 2005;
116:270-73.
90. Hatton DD, Schwietz E, Boyer B, Rychwalski P. Babies Count: the national registry for children with visual impairments,
birth to 3 years. J AAPOS 2007; 11:351-55.
91. Tornqvist K, Ericsson A, Kallen B. Optic nerve hypoplasia: risk factors and epidemiology. Acta Ophthalmol Scand 2002;
80:300-4.
92. Borchert M. Reappraisal of the optic nerve hypoplasia syndrome. J Neuroophthalmol 2012; 32:58-67.
93. Garcia-Filion P, Borchert M. Optic nerve hypoplasia syndrome: a review of the epidemiology and clinical associations. Curr
Treat Options Neurol 2013; 15:78-89.
94. Wong VC. Cortical blindness in children: a study of etiology and prognosis. Pediatr Neurol 1991; 7:178-85.
95. Fazzi E, Bova SM, Uggetti C, et al. Visual-perceptual impairment in children with periventricular leukomalacia. Brain Dev
2004; 26:506-12.

46
96. Hoyt CS. Brain injury and the eye. Eye (Lond) 2007; 21:1285-89.
97. Centers for Disease Control and Prevention. Vision Health Initiative. http://www.cdc.gov/visionhealth/risk/age.htm.
Accessed 2/17/2016.
98. Frazier M, Garces I, Scarinci I, Marsh-Tootle W. Seeking eye care for children: perceptions among Hispanic immigrant
parents. J Immigr Minor Health 2009; 11:215-21.
99. Zhang X, Elliott MN, Saaddine JB, et al. Unmet eye care needs among U.S. 5th-grade students. Am J Prev Med 2012;
43:55-58.
100. Wittenborn JS, Zhang X, Feagan CW, et al. The economic burden of vision loss and eye disorders among the United
States population younger than 40 years. Ophthalmology 2013; 120:1728-35.
101. Grisham D, Powers M, Riles P. Visual skills of poor readers in high school. Optometry 2007; 78:542-49.
102. Powers M, Grisham D, Riles P. Saccadic tracking skills of poor readers in high school. Optometry 2008; 79:228-34.
103. Quaid P, Simpson T. Association between reading speed, cycloplegic refractive error, and oculomotor function in reading
disabled children versus controls. Graefes Arch Clin Exp Ophthalmol 2013; 251:169-87.
104. Schmidt P, Maguire M, Dobson V, et al. Comparison of preschool vision screening tests as administered by licensed eye
care professionals in the Vision In Preschoolers Study. Ophthalmology 2004; 111:637-50.
105. National Academies of Sciences, Engineering, and Medicine. Making Eye Health a Population Health Imperative: Vision
for Tomorrow. The National Academies Press, 2016: Washington, D.C.
106. Centers for Disease Control and Prevention. Improving the Nation’s Vision Health: a Coordinated Public Health
Approach. https://stacks.cdc.gov/view/cdc/6846. Accessed 10/20/2016.
107. U.S. Preventive Services Task Force. Vision screening for children 1 to 5 years of age: US Preventive Services Task Force
Recommendation statement. Pediatrics 2011; 127:340-46.
108. Chou R, Dana T, Bougatsos C. Screening for visual impairment in children ages 1-5 years: update for the USPSTF.
Pediatrics 2011; 127:e442-79.
109. Ying GS, Kulp MT, Maguire M, et al. Sensitivity of screening tests for detecting vision in preschoolers-targeted vision
disorders when specificity is 94%. Optom Vis Sci 2005; 82:432-38.
110. Vision in Preschoolers Study Group. Preschool vision screening tests administered by nurse screeners compared with
lay screeners in the Vision in Preschoolers Study. Invest Ophthalmol Vis Sci 2005; 46:2639-48.
111. Lieberman S, Cohen AH, Stolzberg M, Ritty JM. Validation study of the New York State Optometric Association (NYSOA)
Vision Screening Battery. Am J Optom Physiol Opt 1985; 62:165-68.
112. Bodack MI, Chung I, Krumholtz I. An analysis of vision screening data from New York City public schools. Optometry
2010; 81:476-84.
113. Jacobson J. Why can’t Johnny read? Abell Report 2010; 23:1-8.
114. Hartmann EE, Block SS, Wallace DK. Vision and eye health in children 36 to <72 months: proposed data system.
Optom Vis Sci 2015; 92:24-30.

47
115. Jones-Jordan LA, Sinnott LT, Manny RE, et al. Early childhood refractive error and parental history of myopia as
predictors of myopia. Invest Ophthalmol Vis Sci 2010; 51:115-21.
116. Kurtz D, Hyman L, Gwiazda JE, et al. Role of parental myopia in the progression of myopia and its interaction with
treatment in COMET children. Invest Ophthalmol Vis Sci 2007; 48:562-70.
117. Anstice NS, Thompson B. The measurement of visual acuity in children: an evidence-based update. Clin Exp Optom
2014; 97:3-11.
118. Friedman DS, Katz J, Repka MX, et al. Lack of concordance between fixation preference and HOTV optotype visual
acuity in preschool children: the Baltimore Pediatric Eye Disease Study. Ophthalmology 2008; 115:1796-99.
119. Hakim OM. Association between fixation preference testing and strabismic pseudoamblyopia. J Pediatr Ophthalmol
Strabismus 2007; 44:174-77.
120. Cotter SA, Tarczy-Hornoch K, Song E, et al. Fixation preference and visual acuity testing in a population-based cohort of
preschool children with amblyopia risk factors. Ophthalmology 2009; 116:145-53.
121. Dobson V, Teller DY. Visual acuity in human infants: a review and comparison of behavioral and electrophysiological
studies. Vision Res 1978; 18:1469-83.
122. Gray LG. Avoiding adverse effects of cycloplegics in infants and children. J Am Optom Assoc 1979; 50:465-70.
123. Wickim SM, Amos JF. Chapter 21: Cycloplegic refraction. In Bartlett JD, Jaanus SD, eds. Clinical Ocular Pharmacology,
5th edition. St. Louis: Butterworth-Heinemann; 2008; 343-48.
124. Twelker JD, Mutti DO. Retinoscopy in infants using a near noncycloplegic technique, cyclopegia with tropicamide 1%,
and cycloplegia with cyclopentolate 1%. Optom Vis Sci 2001; 78:215-22.
125. Bartlett JD, Wesson MD, Swiatocha J, Woolley T. Efficacy of a pediatric cycloplegic administered as a spray. J Am
Optom Assoc 1993; 64:617-21.
126. Goodman CR, Hunter DG, Repka MX. A randomized comparison study of drop versus spray topical cycloplegic
application. Binocul Vis Strabismus Q 1999; 14:107-10.
127. Ismail EE, Rouse MW, De Land PN. A comparison of drop instillation and spray application of 1% cyclopentolate
hydrochloride. Optom Vis Sci 1994; 71:235-41.
128. Wesson MD, Bartlett JD, Swiatocha J, Woolley T. Mydriatic efficacy of a cycloplegic spray in the pediatric population. J
Am Optom Assoc 1993; 64:637-40.
129. Syrimi M, Jones SM, Thompson GM. A prospective comparison between cyclopentolate spray and drops in pediatric
outpatients. J Pediatr Ophthalmol Strabismus 2013; 50:290-95.
130. Mohindra I. A technique for infant vision examination. Am J Optom Physiol Opt 1975; 52:867-70.
131. Wesson MD, Mann KR, Bray NW. A comparison of cycloplegic refraction to the near retinoscopy technique for refractive
error determination. J Am Optom Assoc 1990; 61:680-84.
132. Borghi RA, Rouse MW. Comparison of refraction obtained by “near retinoscopy” and retinoscopy under cycloplegia. Am
J Optom Physiol Opt 1985; 62:169-72.

48
133. Anker S, Atkinson J, Braddick O, et al. Identification of infants with significant refractive error and strabismus in a
population screening program using noncycloplegic videorefraction and orthoptic examination. Invest Ophthalmol Vis Sci
2003; 44:497-504.
134. Griffin JR, Cotter SA. The Brückner test: evaluation of clinical usefulness. Am J Optom Physiol Opt 1986; 63:957-61.
135. Gräf M, Jung A. The Brückner test: extended distance improves sensitivity for ametropia. Graefes Arch Clin Exp
Ophthalmol 2008; 246:135-41.
136. Ciner EB, Ying GS, Kulp MT, et al. Stereoacuity of preschool children with and without vision disorders. Optom Vis Sci
2014; 91:351-58.
137. Cyert L, Schmidt P, Maguire M, et al. Threshold visual acuity testing of preschool children using the crowded HOTV and
Lea Symbols acuity tests. J AAPOS 2003; 7:396-99.
138. Vision in Preschoolers Study Group. Preschool visual acuity screening with HOTV and Lea symbols: testability and
between-test agreement. Optom Vis Sci 2004; 81:678-83.
139. Vision in Preschoolers Study Group. Effect of age using Lea Symbols or HOTV for preschool vision screening. Optom Vis
Sci 2010; 87:87-95.
140. Hered RW, Murphy S, Clancy M. Comparison of the HOTV and Lea Symbols charts for preschool vision screening. J
Pediatr Ophthalmol Strabismus 1997; 34:24-28.
141. Choong YF, Chen AH, Goh PP. A comparison of autorefraction and subjective refraction with and without cycloplegia in
primary school children. Am J Ophthalmol 2006; 142:68-74.
142. Vision in Preschoolers Study Group. Comparison of the Retinomax and Palm-AR Auto-Refractors: a pilot study. Optom
Vis Sci 2011; 88:830-36.
143. Kemper AR, Keating LM, Jackson JL, Levin EM. Comparison of monocular autorefraction to comprehensive eye
examinations in preschool-aged and younger children. Arch Pediatr Adolesc Med 2005; 159:435-39.
144. Schmidt PP, Maguire MG, Moore B, Cyert L. Testability of preschoolers on stereotests used to screen vision disorders.
Optom Vis Sci 2003; 80:753-57.
145. Birch E, Williams C, Drover J, et al. Randot Preschool Stereoacuity Test: normative data and validity. J AAPOS 2008;
12:23-26.
146. Pageau M, de Guise D, Saint-Amour D. Random-dot stereopsis in microstrabismic children: stimulus size matters.
Optom Vis Sci 2015; 92:208-16.
147. Fawcett SL, Birch EE. Interobserver test-retest reliability of the Randot preschool stereoacuity test. J AAPOS 2000;
4:354-58.
148. Wesson MD. Normalization of prism bar vergences. Am J Optom Physiol Opt 1982; 59:628-34.
149. McClelland JF, Saunders KJ. The repeatability and validity of dynamic retinoscopy in assessing the accommodative
response. Ophthalmic Physiol Opt 2003; 23:243-50.
150.Tarczy-Hornoch K. Modified bell retinoscopy: measuring accommodative lag in children. Optom Vis Sci 2009; 86:1337-
45.

49
151. Cole BL. The handicap of abnormal colour vision. Clin Exp Optom 2004; 87:258-75.
152. Gnadt GR, Amos JF. Dichromacy and its effect on a young male. J Am Optom Assoc 1992; 63:475-80.
153. Birch J. Efficiency of the Ishihara test for identifying red-green colour deficiency. Ophthalmic Physiol Opt 1997; 17:403-8.
154. Cole BL, Lian KY, Lakkis C. The new Richmond HRR pseudoisochromatic test for colour vision is better than the Ishihara
test. Clin Exp Optom 2006; 89:73-80.
155. Manny RE, Hussein M, Gwiazda J, Marsh-Tootle W. Repeatability of ETDRS visual acuity in children. Invest Ophthalmol
Vis Sci 2003; 44:3294-300.
156. Hopkins S, Sampson GP, Hendicott P, et al. Refraction in children: a comparison of two methods of accommodation
control. Optom Vis Sci 2012; 89:1734-39.
157. Sanfilippo PG, Chu BS, Bigault O, et al. What is the appropriate age cut-off for cycloplegia in refraction? Acta
Ophthalmol 2014; 92:e458-62.
158. Jorge J, Queirós A, Almeida JB, Parafita MA. Retinoscopy/autorefraction: which is the best starting point for a
noncycloplegic refraction? Optom Vis Sci 2005; 82:64-68.
159. Wick B, Hall P. Relation among accommodative facility, lag, and amplitude in elementary school children. Am J Optom
Physiol Opt 1987; 64:593-98.
160. Cole BL. Assessment of inherited colour vision defects in clinical practice. Clin Exp Optom 2007; 90:157-75.
161. Steward JM, Cole BL. What do color vision defectives say about everyday tasks? Optom Vis Sci 1989; 66:288-95.
162. Parisi ML, Scheiman M, Coulter RS. Comparison of the effectiveness of a nondilated versus dilated fundus examination
in the pediatric population. J Am Optom Assoc 1996; 67:266-72.
163. Bansal AS, Hubbard GB 3rd. Peripheral retinal findings in highly myopic children < or =10 years of age. Retina 2010;
30:S15-19.
164. Bradfield YS, Kaminski BM, Repka MX, et al. Comparison of Tono-Pen and Goldmann applanation tonometers for
measurement of intraocular pressure in healthy children. J AAPOS 2012; 16:242-48.
165. Cook JA, Botello AP, Elders A, et al. Systematic review of the agreement of tonometers with Goldmann applanation
tonometry. Ophthalmology 2012; 119:1552-57.
166. Grigorian F, Grigorian AP, Li A, et al. Comparison of the Icare rebound tonometry with the Goldmann applanation
tonometry in a pediatric population. J AAPOS 2015; 19:572-74.
167. Das M, Spowart K, Crossley S, Dutton GN. Evidence that children with special needs all require visual assessment. Arch
Dis Child 2010; 95:888-92.
168. Shevell M, Ashwal S, Donley D, et al. Practice parameter: evaluation of the child with global developmental delay: report
of the Quality Standards Subcommittee of the American Academy of Neurology and The Practice Committee of the Child
Neurology Society. Neurology 2003; 60:367-80.
169. Coulter RA, Bade A, Tea Y, et al. Eye examination testability in children with autism and in typical peers. Optom Vis Sci
2015; 92:31-43.

50
170. Centers for Disease Control and Prevention. Nonfatal traumatic brain injuries from sports and recreation activities –
United States 2001-2005. MMWR Morb Mortal Wkly Rep 2007;56:733-37.
171. Centers for Disease Control and Prevention. Nonfatal traumatic brain injuries related to sports and recreation activities
among persons aged ≤19 years – United States, 2001-2009. MMWR Morb Mortal Wkly Rep 2011; 60:1337-42.
172. Zuckerman SL, Lee YM, Odom MJ, et al. Recovery from sports-related concussion: days to return to neurocognitive
baseline in adolescents versus young adults. Surg Neurol Int 2012; 3:130.
173. Sim A, Terryberry-Spohr L, Wilson KR. Prolonged recovery of memory functioning after mild traumatic brain injury in
adolescent athletes. J Neurosurg 2008; 108:511-16.
174. Moser RS, Schatz P, Jordan BD. Prolonged effects of concussion in high school athletes. Neurosurgery 2005; 57:300-6.
175. Field M, Collins MW, Lovell MR, Maroon J. Does age play a role in recovery from sports-related concussion? A
comparison of high school and collegiate athletes. J Pediatr 2003; 142:546-53.
176. Master CL, Scheiman M, Gallaway M, et al. Vision diagnoses are common after concussion in adolescents. Clin Pediatr
(Phila)2016; 55:260-67.
177. Kivlin JD, Simons KB, Lazoritz S, Ruttum MS. Shaken Baby Syndrome. Ophthalmology 2000; 107:1246-54.
178. Mills M. Funduscopic lesions associated with mortality in Shaken Baby Syndrome. J AAPOS 1998; 2:67-71.
179. Han DP, Wilkinson WS. Late ophthalmic manifestations of the Shaken Baby Syndrome. J Pediatr Ophthalmol Strabismus
1990; 27:299-303.
180. Smith SK. Child abuse and neglect: a diagnostic guide for the optometrist. J Am Optom Assoc 1988; 59:760-66.
181. Budenz DL, Farber MG, Mirchandani HG, et al. Ocular and optic nerve hemorrhages in abused infants with intracranial
injuries. Ophthalmology 1994; 101:559-65.
182. Binenbaum G, Forbes BJ. The eye in child abuse: key points on retinal hemorrhages and abusive head trauma. Pediatr
Radiol 2014; 44 Suppl 4:S571-77.
183: Bhardwaj G, Chowdhury V, Jacobs MB, et al. A systematic review of the diagnostic accuracy of ocular signs in pediatric
abusive head trauma. Ophthalmology 2010; 117:983-92.
184. Classé JG. Release of spectacle prescriptions: an update. J Am Optom Assoc 1996; 67:631-37.
185. Kemp EC, Floyd MR, McCord-Duncan E, Lang F. Patients prefer the method of “tell back-collaborative inquiry” to assess
understanding of medical information. J Am Board Fam Med 2008; 21:24-30.
186. Brand PL, Stiggelbout AM. Effective follow-up consultations: the importance of patient-centered communication and
shared decision making. Paediatr Respir Rev 2013; 14:224-28.
187. Yin HS, Johnson M, Mendelsohn AL, et al. The health literacy of parents in the United States: a nationally representative
study. Pediatrics 2009; 124 Suppl 3:S289-98.
188. Muir KW, Christensen L, Bosworth HB. Health literacy and glaucoma. Curr Opin Ophthalmol 2013; 24:119-24.
189. Hironaka LK, Paasche-Orlow MK. The implications of health literacy on patient-provider communication. Arch Dis Child
2008; 93:428-32.

51
190. Court H, Greenland K, Margrain TH. Predicting state anxiety in optometric practice. Optom Vis Sci 2009; 86:1295-302.
191. Dawn AG, Santiago-Turla C, Lee PP. Patient expectations regarding eye care: focus group results. Arch Ophthalmol
2003; 121:762-68.
192. Americans with Disabilities Act. ADA Title III Technical Assistance Manual. http://www.ada.gov/taman3.html. Accessed
2/16/2016.
193. Pollard KA, Xiang H, Smith GA. Pediatric eye injuries treated in US emergency departments, 1990-2009. Clin Pediatr
(Phila) 2012; 51:374-81.
194. Armstrong GW, Kim JG, Linakis JG, et al. Pediatric eye injuries presenting to United States emergency departments:
2001-2007. Graefes Arch Clin Exp Ophthalmol 2013; 251:629-36.
195. Chen AJ, Linakis JG, Mello MJ, Greenberg PB. Epidemiology of infant ocular and periocular injuries from consumer
products in the United States, 2001-2008. J AAPOS 2013; 17:239-42.
196. McGwin G, Jr., Owsley C. Incidence of emergency department-treated eye injury in the United States. Arch Ophthalmol
2005; 123:662-66.
197. Napier SM, Baker RS, Sanford DG, Easterbrook M. Eye injuries in athletics and recreation. Surv Ophthalmol 1996;
41:229-44.
198. Matter KC, Sinclair SA, Xiang H. Use of protective eyewear in U.S. children: results from the National Health Interview
Survey. Ophthalmic Epidemiol 2007; 14:37-43.
199. Lesniak SP, Bauza A, Son JH, et al. Twelve-year review of pediatric traumatic open globe injuries in an urban U.S.
population. J Pediatr Ophthalmol Strabismus 2012; 49:73-79.
200. Brophy M, Sinclair SA, Hostetler SG, Xiang H. Pediatric eye injury-related hospitalizations in the United States. Pediatrics
2006; 117:e1263-71.
201. Boettner EA, Wolter JR. Transmission of the ocular media. Invest Ophthalmol Vis Sci 1962; 6:776-83.
202. Barker FM, Brainard GC. The direct spectral transmittance of the excised human lens as a function of age. US Food and
Drug Administration Report 1991.FDA 785345 0090 Washington, DC.
203. Lucas RM. An epidemiological perspective of ultraviolet exposure–public health concerns. Eye Contact Lens 2011;
37:168-75.
204. Sui GY, Liu GC, Liu GY, et al. Is sunlight exposure a risk factor for age-related macular degeneration? A systematic
review and meta-analysis. Br J Ophthalmol 2013; 97:389-94.
205. Okuno T. Hazards of solar blue light. Appl Opt 2008; 47:2988-92.
206. Wu J, Seregard S, Algvere PV. Photochemical damage of the retina. Surv Ophthalmol 2006; 51:461-81.
207. van der Lely S, Frey S, Garbazza C, et al. Blue blocker glasses as a countermeasure for alerting effects of evening light-
emitting diode screen exposure in male teenagers. J Adolesc Health 2015; 56:113-19.
208. Gwiazda J, Deng L, Manny R, Norton TT. Seasonal variations in the progression of myopia in children enrolled in the
Correction of Myopia Evaluation Trial. Invest Ophthalmol Vis Sci 2014; 55:752-58.

52
209. Jones-Jordan LA, Mitchell GL, Cotter SA, et al. Visual activity before and after the onset of juvenile myopia. Invest
Ophthalmol Vis Sci 2011; 52:1841-50.
210. Jones-Jordan LA, Sinnott LT, Cotter SA, et al. Time outdoors, visual activity, and myopia progression in juvenile-onset
myopes. Invest Ophthalmol Vis Sci 2012; 53:7169-75.
211. Lin Z, Vasudevan B, Jhanji V, et al. Near work, outdoor activity, and their association with refractive error. Optom Vis Sci
2014; 91:376-82.
212. Rose KA, Morgan IG, Ip J, et al. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology 2008;
115: 1279-85.
213. Morgan IG, Ohno-Matsui K, Saw SM. Myopia. Lancet 2012; 379:1739-48.
214. Turnbull PR, Munro OJ, Phillips JR. Contact lens methods for clinical myopia control. Optom Vis Sci 2016; 93:1120-26.
215. Chia A, Lu QS, Tan D. Five-year clinical trial on atropine for the treatment of myopia 2: myopia control with atropine
0.01% eyedrops. Ophthalmology 2016; 123:391-99.
216. Liu YM, Xie P. The safety of orthokeratology—a systematic review. Eye Contact Lens 2016; 42:35-42.
217. Lin HJ, Wan L, Tsai FJ, et al. Overnight orthokeratology is comparable with atropine in controlling myopia. BMC
Ophthalmol 2014; 14:40.
218. Silva PS, Cavallerano JD, Aiello LM, Aiello LP. Telemedicine and diabetic retinopathy: moving beyond retinal screening.
Arch Ophthalmol 2011; 129:236-42.
219. Gwiazda J, Brill S, Mohindra I, Held R. Preferential looking acuity in infants from two to fifty-eight weeks of age. Am J
Optom Physiol Opt 1980; 57:428-32.
220. Banks MS. The development of visual accommodation during early infancy. Child Dev 1980; 51:646-66.
221. Brookman KE. Ocular accommodation in human infants. Am J Optom Physiol Opt 1983; 60:91-99.
222. Banks MS, Aslin RN, Letson RD. Sensitive period for the development of human binocular vision. Science 1975;
190:675-77.
223. Hohmann A, Creutzfeldt OD. Squint and the development of binocularity in humans. Nature 1975; 254:613-14.
224. Ciner EB, Scheiman MM, Schanel-Klitsch E, Weil L. Stereopsis testing in 18- to 35-month-old children using operant
preferential looking. Optom Vis Sci 1989; 66:782-87.
225. von Noorden GK, Crawford ML. The sensitive period. Trans Ophthalmol Soc U K 1979; 99:442-46.
226. Petrig B, Julesz B, Kropfl W, et al. Development of stereopsis and cortical binocularity in human infants:
electrophysiological evidence. Science 1981; 213:1402-5.
227. Mohindra I, Jacobson SG, Held R. Binocular visual form deprivation in human infants. Doc Ophthalmol 1983; 55:237-49.
228. Hård AL, Niklasson A, Svensson E, Hellström A. Visual function in school-aged children born before 29 weeks of
gestation: a population-based study. Dev Med Child Neurol 2000; 42:100-5.

53
229. Wang J, Ren X, Shen L, et al. Development of refractive error in individual children with regressed retinopathy of
prematurity. Invest Ophthalmol Vis Sci 2013; 54:6018-24.
230. Eibschitz-Tsimhoni M, Friedman T, Naor J, et al. Early screening for amblyogenic risk factors lowers the prevalence and
severity of amblyopia. J AAPOS 2000; 4:194-99.
231. Atkinson J, Braddick O, Nardini M, Anker S. Infant hyperopia: detection, distribution, changes and correlates-outcomes
from the Cambridge infant screening programs. Optom Vis Sci 2007; 84:84-96.
232. Anker S, Atkinson J, Braddick O, et al. Non-cycloplegic refractive screening can identify infants whose visual outcome at
4 years is improved by spectacle correction. Strabismus 2004; 12:227-45.
233. Jones-Jordan L, Wang X, Scherer RW, Mutti DO. Spectacle correction versus no spectacles for prevention of strabismus
in hyperopic children. Cochrane Database Syst Rev 2014: CD007738.
234. McKean-Cowdin R, Varma R, Cotter SA, et al. Risk factors for astigmatism in preschool children: the Multi-Ethnic
Pediatric Eye Disease and Baltimore Pediatric Eye Disease Studies. Ophthalmology 2011; 118:1974-81.
235. Huang J, Maguire MG, Ciner E, et al. Risk factors for astigmatism in the Vision in Preschoolers Study. Optom Vis Sci
2014; 91:514-21.
236. Borchert MS, Varma R, Cotter SA, et al. Risk factors for hyperopia and myopia in preschool children: the Multi-Ethnic
Pediatric Eye Disease and Baltimore Pediatric Eye Disease Studies. Ophthalmology 2011; 118:1966-73.
237. Atkinson J, Braddick O, Robier B, et al. Two infant vision screening programmes: prediction and prevention of
strabismus and amblyopia from photo- and videorefractive screening. Eye (Lond) 1996; 10:189-98.
238. Kulp MT, Ciner E, Maguire M, et al. Uncorrected hyperopia and preschool early literacy: results of the Vision in
Preschoolers-Hyperopia in Preschoolers (VIP-HIP) study. Ophthalmology 2016;123:681-89.
239. Orlansky G, Wilmer J, Taub MB, et al. Astigmatism and early academic readiness in preschool children. Optom Vis Sci
2015; 92:279-85.
240. Dobson V, Clifford-Donaldson CE, Green TK, et al. Optical treatment reduces amblyopia in astigmatic children who
receive spectacles before kindergarten. Ophthalmology 2009; 116:1002-8.
241. Kemper AR, Wallace DK, Patel N, Crews JE. Preschool vision testing by health providers in the United States: findings
from the 2006-2007 Medical Expenditure Panel Survey. J AAPOS 2011; 15:480-83.
242. Comet Group. Myopia stabilization and associated factors among participants in the Correction of Myopia Evaluation
Trial (COMET). Invest Ophthalmol Vis Sci 2013; 54:7871-84.
243. Shankar S, Evans MA, Bobier WR. Hyperopia and emergent literacy of young children: pilot study. Optom Vis Sci 2007;
84:1031-38.
244. van Rijn LJ, Krijnen JS, Nefkens-Molster AE, et al. Spectacles may improve reading speed in children with hyperopia.
Optom Vis Sci 2014; 91:397-403.
245. Borsting E, Rouse MW, Deland PN, et al. Association of symptoms and convergence and accommodative insufficiency
in school-age children. Optometry 2003; 74:25-34.

54
246. Kulp MT, Schmidt PP. Effect of oculomotor and other visual skills on reading performance: a literature review. Optom Vis
Sci 1996; 73:283-92.
247. Kulp MT, Schmidt PP. The relation of clinical saccadic eye movement testing to reading in kindergarteners and first
graders. Optom Vis Sci 1997; 74:37-42.
248. Solan HA, Larson S, Shelley-Tremblay J, et al. Role of visual attention in cognitive control of oculomotor readiness in
students with reading disabilities. J Learn Disabil 2001; 34:107-18.
249. Convergence Insufficiency Treatment Trial Study Group. Randomized clinical trial of treatments for symptomatic
convergence insufficiency in children. Arch Ophthalmol 2008; 126:1336-49.
250. Granet DB, Gomi CF, Ventura R, Miller-Scholte A. The relationship between convergence insufficiency and ADHD.
Strabismus 2005; 13:163-68.
251. Rouse M, Borsting E, Mitchell GL, et al. Academic behaviors in children with convergence insufficiency with and without
parent-reported ADHD. Optom Vis Sci 2009; 86:1169-77.

55
V. APPENDIX
A. APPENDIX FIGURE 1:
Comprehensive Pediatric Eye and Vision Examination: A Flowchart
Final copy 3/3/17
2554
V. APPENDIX 2555
2556
A. Appendix Figure 1 2557
2558
Comprehensive Pediatric Eye and Vision Examination: 2559
A Flowchart 2560
2561
2562
2563
2564
2565
2566
2567
2568
2569
2570
2571

56
B. APPENDIX TABLE 1
Potential Components of the Comprehensive Eye and Vision Examination for Infants and Toddlers
A. Patient History
1. Nature and history of the presenting problem, including chief complaint
2. Visual and ocular history
3. General health history, including prenatal, perinatal, and postnatal history and review of systems, surgical
and/or head or ocular trauma history, and any vision or ocular treatment
4. Medication reconciliation, including prescription and nonprescription drugs (e.g., over the counter
medications, supplements, herbal remedies) and documentation of medication allergies
5. Family ocular and medical histories
6. Developmental history of the child
7. Time spent outdoors, on sports activities, and on near work and screen viewing
8. Names of, and contact information for, the patient’s other health care providers
B. Visual Acuity
1. Preferential looking visual acuity
2. Fixation preference test
3. Visual evoked potential
C. Refraction
1. Cycloplegic retinoscopy
2. Non-cycloplegic retinoscopy
D. Binocular Vision and Ocular Motility
1. Ocular alignment assessment (e.g., cover test, Hirschberg test, Krimsky test)
2. Brückner test
3. Stereopsis (e.g., Preschool Assessment of Stereopsis with a Smile 3 test)
4. Near point of convergence
5. Ocular motility assessment (e.g., versions, eye tracking)
E. Ocular and Systemic Health Assessment
1. Assessment of pupillary responses
2. Visual field evaluation (e.g., confrontation)
3. Evaluation of the ocular anterior segment and adnexa
4. Evaluation of the ocular posterior segment
5. Measurement of intraocular pressure

57
C. APPENDIX TABLE 2
Potential Components of the Comprehensive Eye and Vision Examination for Preschool Children
A. Patient History
1. Nature and history of the presenting problem, including chief complaint
2. Visual and ocular history
3. General health history, including prenatal, perinatal, and postnatal history and review of systems, surgical
and/or head or ocular trauma history, and any vision or ocular treatment
4. Medication reconciliation, including prescription and nonprescription drugs (e.g., over the counter
medications, supplements, herbal remedies) and documentation of medication allergies
5. Family eye and medical histories
6. Developmental history of the child
7. Time spent outdoors, on sports activities, and on near work and screen viewing
8. Names of, and contact information for, the patient’s other health care providers
B. Visual Acuity
1. Symbol optotype or letter matching visual acuity measurement
C. Refraction
1. Static (distance) retinoscopy
2. Cycloplegic retinoscopy
3. Autorefraction
D. Binocular Vision, Ocular Motility, and Accommodation
1. Ocular alignment assessment - distance and near (e.g., cover test, Hirschberg test, Krimsky test)
2. Ocular motility assessment
3. Near point of convergence
4. Stereopsis (e.g., Preschool Assessment of Stereopsis with a Smile 3 test, Randot Preschool test)
5. Positive and negative fusional vergence ranges
6. Accommodative testing (e.g., dynamic retinoscopy)
E. Color vision testing
F. Ocular and Systemic Health Assessment
1. Assessment of pupillary responses
2. Visual field evaluation (e.g., confrontation)
3. Evaluation of the ocular anterior segment and adnexa
4. Evaluation of the ocular posterior segment
5. Measurement of intraocular pressure

58
D. APPENDIX TABLE 3
Potential Components of the Comprehensive Eye and Vision Examination for School-age Children
A. Patient History
1. Nature and history of the presenting problem, including chief complaint
2. Visual and ocular history
3. General health history, including prenatal, perinatal, and postnatal history and review of systems, surgical
and/or head or ocular trauma history, and any vision or ocular treatment
4. Medication reconciliation, including prescription and nonprescription drugs (e.g., over the counter
medications, supplements, herbal remedies) and documentation of medication allergies
5. Family eye and medical histories
6. Developmental history of the child
7. School performance history
8. Time spent outdoors, on sports activities, and on near work and screen viewing
9. Names of, and contact information for, the patient’s other health care providers
B. Visual Acuity
1. Snellen visual acuity
2. ETDRS visual acuity
C. Refraction
1. Static (distance) retinoscopy
2. Cycloplegic retinoscopy
3. Subjective refraction
4. Autorefraction
D. Binocular Vision, Ocular Motility, and Accommodation
1. Ocular alignment assessment - distance and near (e.g., cover test, Hirschberg test, Krimsky test, Von Graefe
phoria, Modified Thorington, Maddox Rod)
2. Ocular motility assessment (e.g., fixation, saccades, pursuits)
3. Near point of convergence
4. Stereopsis (e.g., Random dot stereopsis test)
5. Positive and negative fusional vergence ranges
6. Accommodative testing (e.g., amplitude, facility, and response)
E. Color Vision Testing
F. Ocular and Systemic Health Assessment
1. Assessment of pupillary responses
2. Visual field evaluation (e.g., confrontation)
3. Evaluation of the ocular anterior segment and adnexa
4. Evaluation of the ocular posterior segment
5. Measurement of intraocular pressure

59
E. APPENDIX TABLE 4
Partial Listing of Ocular Manifestations of Neurodevelopmental Disorders and Other Syndromes
Neurodevelopmental Disorders Etiology Associated Ocular Manifestations
Aicardi Syndrome Dysgenesis of the corpus callosum Chorioretinal lacunae, optic nerve colobomas,
optic nerve hypoplasia
Alport Syndrome Irregular synthesis of collagen Fleck retinal dystrophy, anterior lenticonus,
corneal dystrophy, cataracts
Angelman Syndrome Deletion of maternal genetic material on
chromosome 15
Strabismus, hypopigmentation of the choroid
Attention Deficit/Hyperactivity Disorder Genetic influences on dopaminergic systems,
prenatal factors such as maternal use of drugs
and alcohol
Convergence insufficiency, accommodative
dysfunction, oculomotor disorders
Autism Spectrum Disorders Unknown; possible link to environmental
stressors, genetic mutations and inflammatory
processes
Deficits in visual acuity, stereoacuity and ocular
alignment; poor saccades and pursuits
Bardet-Biedl Syndrome Mutation in 14 different genes that lead to
problems with the function of cilia in cell
structures
Reduced visual acuity, problems with night vision,
tunnel vision
Batten-Mayou Syndrome Autosomal recessive disorder resulting in
accumulation of lipids
Lipofuscin accumulation in the retina, optic
atrophy, macular pigment
Behçet’s Disease Postulated to be episodic hyperactivity of immune
system
Uveitis, cataracts, optic atrophy, macular edema
Behr Syndrome Autosomal recessive disease resulting in
progressive deterioration of the nervous system
Optic atrophy, retrobulbar neuritis, nystagmus
Branchial Arch Syndrome Disruption of neural crest cell migration Strabismus, proptosis from poorly formed orbits,
coloboma of the eyelid
Cerebral Palsy Disorder of movement and posture secondary to
damage to motor control connections
Strabismus, nystagmus, optic nerve pallor,
cataracts, myopia, accommodative dysfunction
Cerebro-oculo-facial Syndrome Autosomal recessive disorder resulting in
defective swallowing mechanism
Microphthalmia, involuntary eye movements,
congenital cataracts, blepharophimosis
Charot-Marie-Tooth Syndrome Genetic anomaly resulting in progressive muscular
atrophy
Nystagmus, diminished visual acuity
CHARGE Syndrome Common mutation of chromosome 8 resulting in
association of multiple systemic defects
Bilateral retinal coloboma involving the optic
nerve, strabismus, amblyopia
Cri-du-chat Syndrome Deletion of short arm of chromosome 5 Strabismus, hypertelorism, slanting of the
palpebral fissure
Dandy-Walker syndrome Absence of the cerebellar vermis and dilation of
fourth ventricle
Papilledema often seen with hydrocephalus,
ptosis and strabismus secondary to cranial nerve
palsy
de Lange Syndrome Mutation in genes responsible for chromosomal
adhesions
Long eyelashes, ptosis telecanthus, alternating
exotropia

60
Neurodevelopmental Disorders Etiology Associated Ocular Manifestations
Down Syndrome Triplicate 21st chromosome Epicanthal folds, upslanting palpebral fissure,
high refractive error, strabismus, keratoconus,
blepharitis, accommodative dysfunction/
insufficiency
Dubowitz Syndrome Unknown etiology Strabismus, ptosis, telecanthus, epicanthal folds
Ehlers-Danlos Syndrome Genetic or nutritional defects that have altered the
biosynthesis of collagen
Lens subluxation, palpebral skin laxity,
keratoconus, myopia, blue sclera, angiod streaks
Fabry Disease Inherited disorder resulting from an abnormal
build-up of fat in the blood vessel walls
throughout the body
Corneal opacity
Fetal Alcohol Syndrome CNS damage secondary to alcohol crossing the
blood-brain barrier
Telecanthus, strabismus, optic nerve hypoplasia,
ptosis, microphthalmia
Fragile X Syndrome Gene (FMR1) on the X chromosome fails to
allow protein synthesis necessary for neural
development
Strabismus, astigmatism, amblyopia
Gaucher Disease Lysomal storage disease Strabismus, gaze palsies, corneal clouding,
pinguecula
Hunter Syndrome Mucopolysaccharidosis l – Lysomal storage
disease
Corneal clouding, pigmentary degeneration of the
retina, optic atrophy
Lowe Syndrome Abnormal protein transport within cellular
membranes
Bilateral congenital cataracts, glaucoma, corneal
keloids, strabismus
Marfan Syndrome Genetic disorder affecting the body’s connective
tissue
Severe nearsightedness, dislocated lens,
detached retina, glaucoma, cataracts
Prader-Willi Syndrome Deletion of paternal genetic material on
chromosome 15
Strabismus, almond-shaped palpebral fissures,
myopia
Rett Syndrome Mutation of binding protein (MECP2) that alters
the development of gray matter
Difficulty maintaining eye contact
Septo-Optic Dysplasia/DeMorsier Syndrome Disorder of early brain/optic nerve development
associated with a number of environmental and
genetic factors
Visual impairment in one or both eyes, nystagmus,
strabismus
Spina Bifida Incomplete closure of embryonic neural tube Papilledema, nerve palsies, nystagmus, optic
atrophy
Stickler Syndrome Defective biosynthesis of collagen Myopia, retinal detachments, vitreous anomalies
Usher Syndrome Inherited autosomal recessive trait Retinitis pigmentosa
Williams Syndrome Vast deletion of genes on chromosome 7 Infantile esotropia, anomaly in visual-spatial
relationship
Source: Adapted from Table 7.1 Rare Neurodevelopmental Disorders in Taub MB, Bartuccio M, Maino DM.
Visual Diagnosis and Care of the Patient with Special Needs. Lippincott Williams & Wilkins, Philadelphia, PA,
2012.

61
F. ABBREVIATIONS/ACRONYMS
ADA Americans with Disabilities Act
AD/HD Attention Deficit/Hyperactivity Disorder
AHRQ Agency for Healthcare Research and Quality
COI Conflict of interest
CE Convergence excess
CI Convergence insufficiency
CLEERE Collaborative Longitudinal Evaluation of Ethnicity and Refractive Error
CPG Clinical Practice Guideline
CT Computerized tomography
CVI Cortical (cerebral) visual impairment
D Diopter
DR Diabetic retinopathy
EBO Evidence-Based Optometry
ERG Electroretinogram
ETDRS Early Treatment of Diabetic Retinopathy Study
G Grams
GAT Goldmann applanation tonometer
GDG Guideline Development Group
GDRG Guideline Development Reading Group
IDEA Individuals with Disabilities Education Act
IEP Individualized Education Program
IOM Institute of Medicine
IOP Intraocular pressure
MRI Magnetic resonance imaging
NPC Near point of convergence
NRA Negative relative accommodation
OCT Optical coherence tomography
PASS Preschool Assessment of Stereopsis with a Smile
PRA Positive relative accommodation
RCT Randomized clinical trial
ROP Retinopathy of prematurity
RP Retinitis pigmentosa
SE Spherical equivalent
SR Systematic review
TOPEL Test of Preschool Early Literacy
VEP Visual evoked potential
UV Ultraviolet
VIP-HIP Vision in Preschoolers-Hyperopia in Preschoolers

62
G. SUMMARY OF ACTION STATEMENTS
A comprehensive pediatric eye and vision examination should include, but is not limited to:
• Review of the nature and history of the presenting problem, patient and family eye and medical histories,
including visual, ocular, general health, leisure and sports activities, and developmental and school performance
history of the child
• Measurement of visual acuity
• Determination of refractive status
• Assessment of binocular vision, ocular motility, and accommodation
• Evaluation of color vision (baseline or periodic, if needed, for qualification purposes or if disease related)
• Assessment of ocular and systemic health, including evaluation of pupillary responses, anterior and posterior
segment, peripheral retina, and evaluation/measurement of intraocular pressure and visual field testing.
(Consensus)
Cycloplegic retinoscopy is the preferred procedure for the first evaluation of preschoolers. It is necessary to quantify
significant refractive error in the presence of visual conditions such as strabismus, amblyopia, and anisometropia.
(Consensus)
Cycloplegic retinoscopy is the preferred procedure for the first evaluation of school-age children. It is necessary
to quantify significant refractive error in the presence of visual conditions such as strabismus, amblyopia, and
anisometropia. (Consensus)
Abnormal color vision can affect daily performance of activities involving color discrimination and may interfere
with or prevent some occupational choices later in life. Children should be tested as soon as possible for color
vision deficiency and the parents/caregivers of children identified with color vision deficiency should be counseled.
(Consensus)
Children at risk for learning-related vision problems should be evaluated by a doctor of optometry. (Consensus)
Many children with developmental or intellectual disabilities have undetected and untreated vision problems and
should receive a comprehensive pediatric eye and vision examination. (Consensus)
At the conclusion of a comprehensive pediatric eye and vision examination, the diagnosis should be explained to
the patient/parent/caregiver and related to the patient’s symptoms, and a treatment plan and prognosis discussed.
(Consensus)

63
Parents/caregivers and children should be educated about potential risks for eye injuries at home, at school,
and during sports and recreational activities, and advised about safety precautions to decrease the risk of ocular
injury.
193,199
Prevention of eye injuries in children should focus on the use of protective eyewear, parental supervision,
and include education about both the risks of eye injury and the benefits of protective eyewear.
194
(Evidence Grade B/
Strong Recommendation)
All children and their parents/caregivers should be advised about the benefits of the regular use of sunglasses and/
or clear prescription glasses that effectively block at least 99% of UVA and UVB radiation, the use of hats with brims
when outdoors, and the importance of not looking directly at the sun. (Consensus)
Patients/parents/caregivers should be counseled about the benefits to children’s vision of spending more time
outdoors.
208-211
(Evidence Grade B/Recommendation)
Infants should receive an in-person comprehensive eye and vision assessment between 6 and 12 months of age
for the prevention and/or early diagnosis and treatment of sight-threatening eye conditions and to evaluate visual
development.
229-231
(Evidence Grade B/Strong Recommendation)
Preschool age children should receive an in-person comprehensive eye and vision examination at least once
between the ages of 3 and 5 to prevent and/or diagnose and treat any eye or vision conditions that may affect visual
development.
54,107,238,240,241
(Evidence Grade B/Strong Recommendation)
School-age children should receive an in-person comprehensive eye and vision examination before beginning
school to diagnose, treat and manage any eye or vision conditions.
65,115,238,243,244,251
(Evidence Grade B/Strong
Recommendation)
Children with myopia should have an in-person comprehensive eye and vision examination at least annually, or as
frequently as recommended (especially until age 12), because of the potential for rapid myopia progression.
208,242
(Evidence Grade B/Strong Recommendation)
School-age children should receive an in-person comprehensive eye and vision examination annually to diagnose,
treat, and manage eye or vision problems. (Consensus)

64
H. GAPS IN RESEARCH EVIDENCE
During the course of the development of this guideline, the Evidence-Based Optometry Guideline Development
Group identified the following gaps in evidence as potential areas for future research:
• Research to compare the outcomes of vision screenings versus comprehensive eye and vision examinations
• Research to determine the risks and protective factors associated with eye injuries in children in order to design
appropriate prevention strategies
• Research on the effects and possible interaction of outdoor activity with near work and myopia in children.
VI. METHODOLOGY FOR GUIDELINE DEVELOPMENT
This guideline was developed by the AOA Evidence-Based Optometry Guideline Development Group (GDG). Clinical
questions to be addressed in the guideline were identified and refined during an initial meeting of the GDG and served
as the basis for a search of the clinical and research literature.
An English language search of the medical literature for the eye and vision examination of children birth through 18
years of age, for the time period January 2005 through October 2016 was conducted by trained researchers. If the
search did not produce results, the search parameters were extended an additional 5 years.
Search Inclusion Criteria (must meet all):
1. English Studies
2. Study addresses the clinical question(s)
3. Paper meets the age group being addressed (0 to 18 years for pediatrics)
4. Searched by question(s) formulated at the AOA Call to Question Meeting attended by the Guideline
Development Group (GDG)
5. Using all similar and relevant terms as defined by the GDG
Exclusion Criteria (meeting any of the below):
1. Non-English studies
2. Animal studies
3. Studies outside of the patient age range
4. Studies not addressing any topic of the clinical questions searched
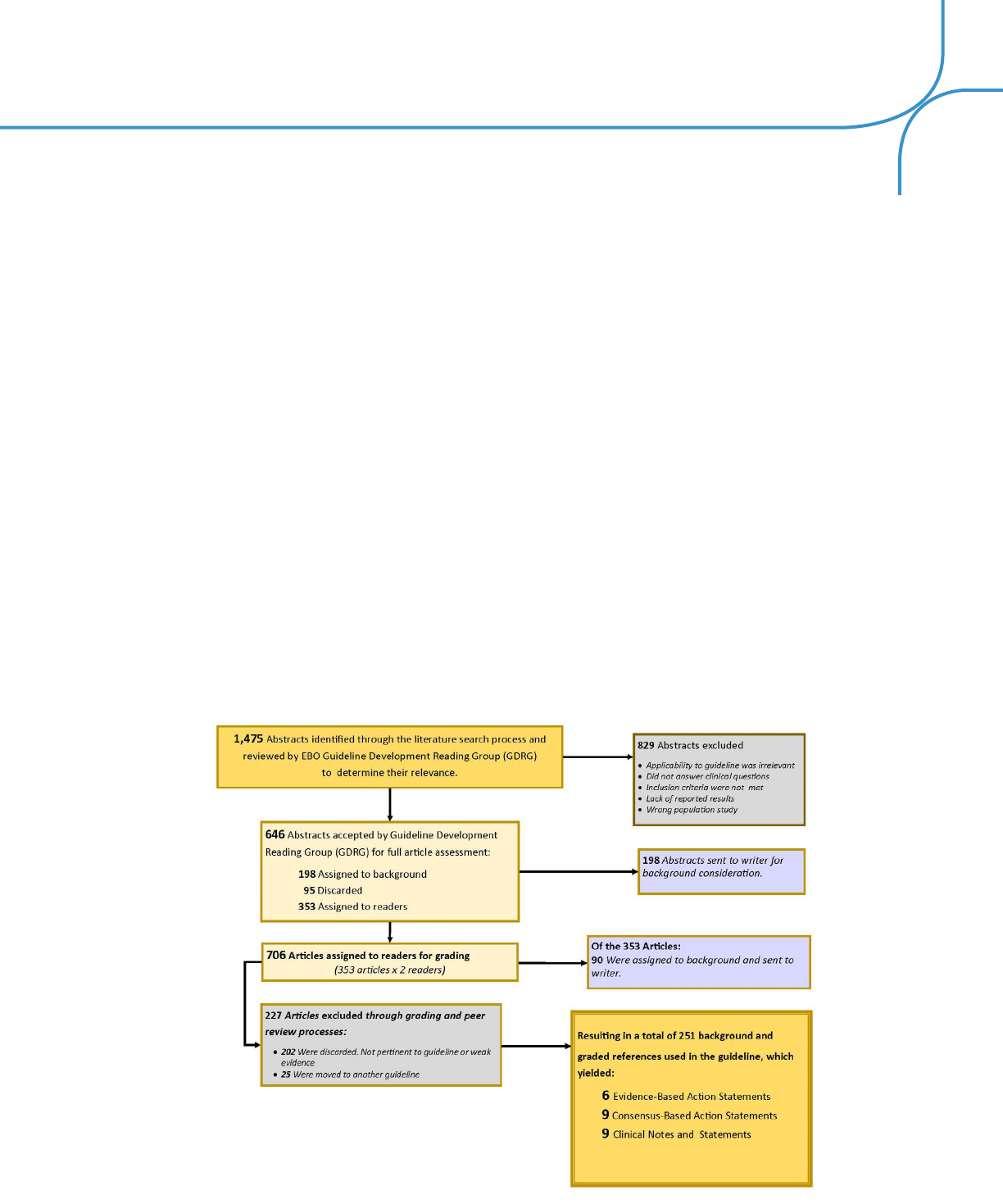
65
In addition, a review of selected earlier research publications was conducted based on previous versions of this
guideline. The literature search was conducted using the following electronic databases:
The literature search resulted in the retrieval of the number of references shown in the following chart.
Final copy 3/3/17
2942
2943
All references meeting the criteria were reviewed to determine their relevance to the clinical questions 2944
addressed in the guideline. Each article was assigned to two clinicians who independently reviewed 2945
and graded the quality of evidence and the clinical recommendations derived from the article, based 2946
on a previously defined system for grading quality. If discrepancies were found in the grading results, 2947
the article was assigned to an independent third reader for review and grading. 2948
During six articulation meetings (three face-to-face and three using a Webex platform) of the 2949
Evidence-Based Optometry Guideline Development Reading Group (GDRG), all evidence was 2950
reviewed and clinical recommendations were developed. The strength level of clinical 2951
recommendations was based on the quality grade of the research and the potential benefits and 2952
harms of the procedure or therapy recommended. Where high quality evidence to support a 2953
recommendation was weak or lacking, a group consensus was required to approve any consensus 2954
recommendations. 2955
Review and editing of the draft guideline by the Evidence-Based Optometry GDG required one face- 2956
to-face meeting and three additional Draft Reading Meetings using a Webex platform. The final Peer 2957
Review draft was reviewed and approved by the GDG by conference call, then made available for 2958
peer and public review for 30 days for numerous stakeholders (individuals and organizations). 2959
Comments were promoted and encouraged. All suggested revisions were reviewed and, if accepted 2960
by the GDG, incorporated into the guideline. All peer and public comments and all actions (and 2961
inactions) were recorded. 2962
Clinical recommendations in this guideline are evidence-based statements regarding patient care that 2963
are supported by the scientific literature or consensus of professional opinion when no quality 2964
• Agency for Healthcare Research and Quality
(AHRQ)
• American Academy of Optometry (AAO)
• American Academy of Neurology
• American Association for Pediatric
Ophthalmology and Strabismus (AAPOS)
• American Journal of Optometry and
Physiological Optics
• Centers for Disease Control and Prevention,
National Center for Health Statistics
• Cochrane Library
• Developmental Medicine & Child Neurology
(DMCN)
• Elsevier
• Epidemiology
• Google Scholar
• JAMA Ophthalmology
• Journal of Adolescent Health Care (JAHC)
• Medline Plus
• National Eye Institute
• National Institute of Health Public Access (NIH)
• National Guideline Clearinghouse
• Neurology
• Ophthalmic Epidemiology
• Ophthalmology
• PubMed
• Other medical journals meeting the search
criteria will be included in this list when used

66
All references meeting the criteria were reviewed to determine their relevance to the clinical questions addressed
in the guideline. Each article was assigned to two clinicians who independently reviewed and graded the quality of
evidence and the clinical recommendations derived from the article, based on a previously defined system for grading
quality. If discrepancies were found in the grading results, the article was assigned to an independent third reader for
review and grading.
During six articulation meetings (three face-to-face and three using a Webex platform) of the Evidence-Based
Optometry Guideline Development Reading Group (GDRG), all evidence was reviewed and clinical recommendations
were developed. The strength level of clinical recommendations was based on the quality grade of the research
and the potential benefits and harms of the procedure or therapy recommended. Where high quality evidence to
support a recommendation was weak or lacking, a group consensus was required to approve any consensus
recommendations.
Review and editing of the draft guideline by the Evidence-Based Optometry GDG required one face- to-face meeting
and three additional Draft Reading Meetings using a Webex platform. The final Peer Review draft was reviewed and
approved by the GDG by conference call, then made available for peer and public review for 30 days for numerous
stakeholders (individuals and organizations). Comments were promoted and encouraged. All suggested revisions
were reviewed and, if accepted by the GDG, incorporated into the guideline. All peer and public comments and all
actions (and inactions) were recorded.
Clinical recommendations in this guideline are evidence-based statements regarding patient care that are supported
by the scientific literature or consensus of professional opinion when no quality evidence was discovered. The
guideline will be periodically reviewed for new scientific and clinical evidence within 3-5 years.
VII. EVIDENCE-BASED OPTOMETRY GUIDELINE DEVELOPMENT GROUP
AOA Evidence-Based Optometry Committee
Diane T. Adamczyk, O.D., Chair – State University of New York, College of Optometry, New York, New York
John F. Amos, O.D., M.S. – University of Alabama at Birmingham School of Optometry, Birmingham, Alabama, Dean and
Professor Emeritus
Felix M. Barker, II, O.D., M.S. – W. G. (Bill) Hefner VAMC, Salisbury, North Carolina
Benjamin P. Casella, OD – Private Practice – Casella Eye Center, Augusta, Georgia
Linda M. Chous, O.D. – United HealthCare Services, Inc., Minneapolis, Minnesota
Lynn D. Greenspan, O.D. – Salus University, Pennsylvania College of Optometry, Elkins Park, Pennsylvania
Lori L. Grover, O.D., Ph.D. – Health Policy, King-Devick Technologies, Inc., Oakbrook Terrace, Illinois
Tina R. MacDonald, O.D. – The Center for the Partially Sighted, Culver City, California
Harue J. Marsden, O.D., M.S. – Southern California College of Optometry, Marshall B. Ketchum University, Fullerton,
California
David K. Masihdas, O.D. – Utah Eye Associates - The Diabetic Eye Center, Salt Lake City, Utah

67
Bennett McAllister, O.D. – Western University of Health Sciences, College of Optometry, Pomona, California
Trennda L. Rittenbach, O.D. – Palo Alto Medical Foundation/Sutter Health, Sunnyvale, California
Carl J. Urbanski, O.D. – Private Practice, Family Vision Care of Kingston, Kingston, Pennsylvania
Multidisciplinary and Patient Stakeholders
Ida Chung, O.D. – Western University of Health Sciences, College of Optometry, Pomona, California
Beth T. Dessem – Patient Advocate; Missouri CASA Association, Columbia, Missouri
David E. Hartenbach, M.D. – Pediatrician; Creve Coeur Pediatrics, Creve Coeur, Missouri
Janet Hughes – Patient (parent); Vision First Foundation, Lemont, Illinois
Mitchell M. Scheiman, O.D. – Salus University, The Eye Institute of Pennsylvania College of Optometry, Elkins Park,
Pennsylvania
Non-voting Members
Stephen C. Miller, O.D., Chief Editor - Innovative Writing Works, St. Louis, Missouri
Beth A. Kneib, O.D., AOA Director of Clinical Resources, American Optometric Association, St. Louis, Missouri
Andrew Morgenstern, O.D., AOA Consultant for Evidence-Based Optometry, American Optometric Association, Alexandria,
VA
Danette Miller, AOA Manager of Quality Improvement, American Optometric Association, St. Louis, Missouri
Alisa G. Krewet, AOA Quality Improvement Coordinator, American Optometric Association, St. Louis, Missouri
